Calcitonin is a 32 amino acid peptide hormone secreted by PARAFOLLICULAR CELLS (also known as C cells) of the thyroid (or endostyle) in humans and other chordates in the ultimopharyngeal body. It acts to reduce blood calcium (Ca2+), opposing the effects of PARATHYROID HORMONE (PTH). Its importance in humans has not been as well established as its importance in other animals, as its function is usually not significant in the regulation of normal calcium homeostasis. It belongs to the calcitonin-like protein family. Historically calcitonin has also been called thyrocalcitonin.
- GRCh38: Ensembl release 89: ENSG00000110680 – Ensembl, May 2017
- “Human PubMed Reference:”. National Center for Biotechnology Information, U.S. National Library of Medicine.
- Sekiguchi T, Kuwasako K, Ogasawara M, Takahashi H, Matsubara S, Osugi T, et al. (January 2016). “Evidence for Conservation of the Calcitonin Superfamily and Activity-regulating Mechanisms in the Basal Chordate Branchiostoma floridae: Insights Into the Molecular and Functional Evolution in Chordates”. The Journal of Biological Chemistry. 291 (5): 2345–2356. doi:10.1074/jbc.M115.664003. PMC 4732217. PMID 26644465.
- Costoff A. “Sect. 5, Ch. 6: Anatomy, Structure, and Synthesis of Calcitonin (CT)”. Endocrinology: hormonal control of calcium and phosphate. Medical College of Georgia. Archived from the original on September 5, 2008. Retrieved 2008-08-07.
- Boron WF, Boulpaep EL (2004). “Endocrine system chapter”. Medical Physiology: A Cellular And Molecular Approach. Elsevier/Saunders. ISBN 1416023283.
- Costoff A. “Sect. 5, Ch. 6: Biological Actions of CT”. Medical College of Georgia. Archived from the original on July 5, 2008. Retrieved 2008-08-07.
- Felsenfeld, Arnold J.; Levine, Barton S. (April 2015). “Calcitonin, the forgotten hormone: does it deserve to be forgotten?”. Clinical Kidney Journal. 8 (2): 180–187. doi:10.1093/ckj/sfv011. ISSN 2048-8505. PMC 4370311. PMID 25815174.
The ultimopharyngeal body, or ultimobranchial body or ultimobranchial gland is a small organ found in the neck region of many animals. In humans, it develops from the FOURTH PHARYNGEAL POUCH into the parafollicular cells of the thyroid to produce CALCITONIN. It may not develop in DiGeorge syndrome. In humans, it develops into other tissues. In humans, the ultimopharyngeal body is an embryological structure, and is a derivative of the ventral recess of the fourth pharyngeal pouch. It is technically from the fifth pharyngeal pouch, but this is rudimentary and merges with the fourth. It develops into the parafollicular cells of the thyroid. The cells that give rise to the parafollicular cells are derivatives of endoderm. Endoderm cells migrate and associate with the ultimopharyngeal body during development. In humans, the ultimopharyngeal body develops into the parafollicular cells of the thyroid. These secrete CALCITONIN. In other animals, the ultimopharyngeal body may produce calcitonin. Another Wikipedia page says: Another Wikipedia page says: In the embryonic development of vertebrates, pharyngeal pouches form on the endodermal side between the pharyngeal arches. The pharyngeal grooves (or clefts) form the lateral ectodermal surface of the neck region to separate the arches. The pouches line up with the clefts, and these thin segments become GILLS in fish. First pouch – The endoderm lines the future auditory tube (Pharyngotympanic Eustachian tube, middle ear, mastoid antrum, and inner layer of the tympanic membrane. Derivatives of this pouch are supplied by Mandibular nerve. Second pouch – Contributes the middle ear, palantine tonsils, supplied by the facial nerve. Third pouch – The third pouch possesses Dorsal and Ventral wings. Derivatives of the dorsal wings include the inferior parathyroid glands, while the ventral wings fuse to form the cytoreticular cells of the thymus. The main nerve supply to the derivatives of this pouch is Cranial Nerve IX, glossopharyngeal nerve. Fourth pouch – derivatives include: superior parathyroid glands and ultimobranchial body which forms the parafollicular C-Cells of the thyroid gland, musculature and cartilage of larnyx (along with the sixth pharyngeal arch), nerve supplying these derivatives is Superior laryngeal nerve. Fifth pouch – rudimentary structure, becomes part of the fourth pouch contributing to thyroid C-cells. Sixth pouch – The fourth and sixth pouches contribute to the formation of the musculature and cartilage of the larynx. Nerve supply is by the recurrent laryngeal nerve. See also Pharyngeal arch (often called branchial arch although this is more specifically a fish structure), DiGeorge syndrome, List of human cell types derived from the germ layers.
- “Ultimobranchial bodies”“at Dorland’s Medical Dictionary
- Adams A, Mankad K, Offiah C, Childs L (February 2016). “Branchial cleft anomalies: a pictorial review of embryological development and spectrum of imaging findings”. Insights into Imaging. 7 (1): 69–76. doi:10.1007/s13244-015-0454-5. PMC 4729717. PMID 26661849.
- Johansson E, Andersson L, Örnros J, Carlsson T, Ingeson-Carlsson C, Liang S, et al. (October 2015). “Revising the embryonic origin of thyroid C cells in mice and humans”. Development. 142 (20): 3519–3528. doi:10.1242/dev.126581. PMC 4631767. PMID 26395490.
- Agathos EA, Tomos PI, Kostomitsopoulos N, Koutsoukos PG (February 2019). “Calcitonin as an anticalcification treatment for implantable biological tissues”. Journal of Cardiology. New insights in treatment for heart failure. 73 (2): 179–182. doi:10.1016/j.jjcc.2018.07.010. PMID 30377016. S2CID 53110929.
Biosynthesis and regulation
Calcitonin is formed by the PROTEOLYTIC cleavage of a larger prepropeptide, which is the product of the CALC1 gene (CALCA). It is functionally an antagonist with PTH and Vitamin D3. The CALC1 gene belongs to a superfamily of related protein hormone precursors including islet amyloid precursor protein, calcitonin gene-related peptide, and the precursor of adrenomedullin.
Secretion of calcitonin is stimulated by:
- an increase in serum [Ca2+]
- Costanzo, Linda S. (2007). BRS Physiology. Lippincott, Williams, & Wilkins. pp. 263. ISBN 978-0-7817-7311-9.
- GASTRIN and PENTAGASTRIN.
Function
Main article: Calcium metabolism
The hormone participates in calcium (Ca2+) metabolism. In many ways, calcitonin counteracts parathyroid hormone (PTH) and vitamin D.
More specifically, calcitonin lowers blood Ca2+ levels in two ways:
- Major effect: Inhibits OSTEOCLAST activity in bones, which break down the bone
- Costoff A. “Sect. 5, Ch. 6: Effects of CT on Bone”. Medical College of Georgia. Archived from the original on June 22, 2008. Retrieved 2008-08-07.
- Minor effect: Inhibits RENAL TUBULAR CELL reabsorption of Ca2+ and phosphate, allowing them to be excreted in the URINE
- Potts J, Jüppner H (2008). “Chapter 353. Disorders of the Parathyroid Gland and Calcium Homeostasis”. In Longo DL, Kasper DL, Jameson JL, Fauci AS, Hauser SL, Loscalzo J (eds.). Harrison’s Principles of Internal Medicine (18th ed.). McGraw-Hill. Archived from the original on 2017-05-08. Retrieved 2017-05-29.
- Rhoades R (2009). Medical Physiology: Principles for Clinical Medicine. Philadelphia: Lippincott Williams & Wilkins. ISBN978-0781768528.[page needed]
High concentrations of calcitonin may be able to increase urinary excretion of calcium and phosphate via the renal tubules leading to marked HYPOCALCEMIA. However, this is a minor effect with no physiological significance in humans. It is also a short-lived effect because the kidneys become resistant to calcitonin, as demonstrated by the kidney’s unaffected excretion of calcium in patients with thyroid tumors that secrete excessive calcitonin.
- Carney SL (1997). “Calcitonin and human renal calcium and electrolyte transport”. Mineral and Electrolyte Metabolism. 23 (1): 43–47. PMID 9058369.
- Goodman HM (2009). Basic Medical Endocrinology (Fourth ed.). Elsevier. ISBN978-0123739759.[page needed]
In its skeleton-preserving actions, calcitonin protects against calcium loss from the skeleton during periods of calcium mobilization, such as pregnancy and, especially, lactation. The protective mechanisms include the direct inhibition of bone resorption and the indirect effect through the inhibition of the release of prolactin from the pituitary gland. The reason provided is that PROLACTIN induces the release of PTH related peptide which enhances bone resorption, but is still under investigation.
- Horwitz MJ, Tedesco MB, Sereika SM, Syed MA, Garcia-Ocaña A, Bisello A, Hollis BW, Rosen CJ, Wysolmerski JJ, Dann P, Gundberg C, Stewart AF (October 2005). “Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2 vitamin D”. J. Bone Miner. Res. 20 (10): 1792–1803. doi:10.1359/JBMR.050602. PMID 16160737. S2CID 25594820.
- Davey RA, Turner AG, McManus JF, Chiu WS, Tjahyono F, Moore AJ, Atkins GJ, Anderson PH, Ma C, Glatt V, MacLean HE, Vincent C, Bouxsein M, Morris HA, Findlay DM, Zajac JD (August 2008). “Calcitonin receptor plays a physiological role to protect against hypercalcemia in mice”. J. Bone Miner. Res. 23 (8): 1182–1193. doi:10.1359/jbmr.080310. PMC 2680171. PMID 18627265
- Woodrow JP, Sharpe CJ, Fudge NJ, Hoff AO, Gagel RF, Kovacs CS (September 2006). “Calcitonin plays a critical role in regulating skeletal mineral metabolism during lactation”. Endocrinology. 147 (9): 4010–4021. doi:10.1210/en.2005-1616. PMID 16675524.
Other effects are in preventing postprandial hypercalcemia resulting from absorption of Ca2+. Also, calcitonin inhibits food intake in rats and monkeys, and may have CNS action involving the regulation of feeding and appetite.
Calcitonin lowers blood calcium and phosphorus mainly through its inhibition of osteoclasts. OSTEOBLASTS do not have calcitonin receptors and are therefore not directly affected by calcitonin levels. However, since bone resorption and bone formation are coupled processes, eventually calcitonin’s inhibition of osteoclastic activity leads to increased osteoblastic activity (as an indirect effect).
- Goodman HM (2009). Basic Medical Endocrinology (Fourth ed.). Elsevier. ISBN978-0123739759.[page needed]
Receptor
The calcitonin receptor is a G protein-coupled receptor localized to osteoclasts as well kidney and brain cells. It is coupled to a Gsα subunit, thus stimulating cAMP production by ADENYLATE CYCLASE in target cells. It may also affect the ovaries in women and the testes in men.[citation needed]
- Nicholson GC, Moseley JM, Sexton PM, Mendelsohn FA, Martin TJ (August 1986). “Abundant calcitonin receptors in isolated rat osteoclasts. Biochemical and autoradiographic characterization”. J. Clin. Invest. 78 (2): 355–360. doi:10.1172/JCI112584. PMC 423551. PMID 3016026.
Discovery
Calcitonin was first purified in 1962 by Douglas Harold Copp and B. Cheney at the University of British Columbia, Canada.
- Copp DH, Cheney B (January 1962). “Calcitonin-a hormone from the parathyroid which lowers the calcium-level of the blood”. Nature. 193 (4813): 381–2. Bibcode:1962Natur.193..381C. doi:10.1038/193381a0. PMID 13881213. S2CID 4292938.
It was initially thought to be secreted by the parathyroid gland but was shown by Iain Macintyre and his team at the Royal Postgraduate Medical School, London, to be secreted by parafollicular cells of the thyroid gland.
- Foster GV, Baghdiantz A, Kumar MA, Slack E, Soliman HA, Macintyre I (June 1964). “Thyroid origin of calcitonin”. Nature. 202 (4939): 1303–5. Bibcode:1964Natur.202.1303F. doi:10.1038/2021303a0. PMID 14210962. S2CID 2443410.
Dr. Copp named the discovered hormone calcitonin because of its role in ‘maintaining normal calcium tone’.
- Copp DH, Cheney B (January 1962). “Calcitonin-a hormone from the parathyroid which lowers the calcium-level of the blood”. Nature. 193 (4813): 381–2. Bibcode:1962Natur.193..381C. doi:10.1038/193381a0. PMID 13881213. S2CID 4292938.
Medical significance
Calcitonin assay is used in identifying patients with nodular thyroid diseases. It is helpful in making an early diagnosis of medullary carcinoma of thyroid. A malignancy of the parafollicular cells, i.e. Medullary thyroid cancer, typically produces an elevated serum calcitonin level. Prognosis of MTC depends on early detection and treatment.
Calcitonin also has significantly impacted molecular biology, as the gene encoding calcitonin was the first gene discovered in mammalian cells to be alternatively spliced, now known to be a ubiquitous mechanism in eukaryotes.
- Rosenfeld MG, Amara SG, Roos BA, Ong ES, Evans RM (March 1981). “Altered expression of the calcitonin gene associated with RNA polymorphism”. Nature. 290 (5801): 63–65. Bibcode:1981Natur.290…63R. doi:10.1038/290063a0. PMID 7207587. S2CID 4318349.
- Rosenfeld MG, Lin CR, Amara SG, Stolarsky L, Roos BA, Ong ES, Evans RM (March 1982). “Calcitonin mRNA polymorphism: peptide switching associated with alternative RNA splicing events”. Proceedings of the National Academy of Sciences of the United States of America. 79 (6): 1717–1721. Bibcode:1982PNAS…79.1717R. doi:10.1073/pnas.79.6.1717. PMC 346051. PMID 6952224.
Pharmacology
Calcitonin has clinically been used for metabolic bone disorders for more than 50 years.
- das Neves, José; Sarmento, Bruno (2014). Mucosal Delivery of Biopharmaceuticals. Boston: Springer US. pp. 407–422. doi:10.1007/978-1-4614-9524-6_18. ISBN 978-1461495246.
Salmon calcitonin is used for the treatment of:
- Postmenopausal osteoporosis
- “Handout on Health: Osteoporosis”. NIAMS. August 2014. Archived from the original on 18 May 2015. Retrieved 16 May 2015.
- Hypercalcaemia
- “Hypercalcemia”. The Lecturio Medical Concept Library. Retrieved 1 October 2021.
- Bone metastases
- Paget’s disease
- “Paget’s Disease of Bone”. The Lecturio Medical Concept Library. Retrieved 1 October 2021.
- Phantom limb pain
- It has been investigated as a possible non-operative treatment for spinal stenosis.
- Tran DQ, Duong S, Finlayson RJ (July 2010). “Lumbar spinal stenosis: a brief review of the nonsurgical management”. Can J Anaesth. 57 (7): 694–703. doi:10.1007/s12630-010-9315-3. PMID 20428988.
- The following information is from the UK Electronic Medicines Compendium
- “Electronic Medicines Compendium”. Archived from the original on 2005-11-08. Retrieved 2008-08-07.
General characteristics of the active substance
Salmon calcitonin is rapidly absorbed and eliminated. Peak plasma concentrations are attained within the first hour of administration.
Animal studies have shown that calcitonin is primarily metabolised via proteolysis in the kidney following parenteral administration. The metabolites lack the specific biological activity of calcitonin. Bioavailability following subcutaneous and intramuscular injection in humans is high and similar for the two routes of administration (71% and 66%, respectively).
Calcitonin has short absorption and elimination half-lives of 10–15 minutes and 50–80 minutes, respectively. Salmon calcitonin is primarily and almost exclusively degraded in the kidneys, forming pharmacologically inactive fragments of the molecule. Therefore, the metabolic clearance is much lower in patients with end-stage kidney failure than in healthy subjects. However, the clinical relevance of this finding is not known. Plasma protein binding is 30% to 40%.
Characteristics in patients
There is a relationship between the subcutaneous dose of calcitonin and peak plasma concentrations. Following parenteral administration of 100 IU calcitonin, peak plasma concentration lies between about 200 and 400 pg/ml. Higher blood levels may be associated with increased incidence of nausea, vomiting, and secretory diarrhea.
Preclinical safety data
Conventional long-term toxicity, reproduction, mutagenicity, and carcinogenicity studies have been performed in laboratory animals. Salmon calcitonin is devoid of embryotoxic, teratogenic, and mutagenic potential.
- An increased incidence of pituitary adenomas has been reported in rats given synthetic salmon calcitonin for 1 year. This is considered a species-specific effect and of no clinical relevance.
- Salmon calcitonin does not cross the placental barrier.
In lactating animals given calcitonin, suppression of MILK production has been observed. Calcitonin is secreted into the milk.
Pharmaceutical manufacture
Calcitonin was extracted from the ultimobranchial glands (thyroid-like glands) of fish, particularly salmon. Salmon calcitonin resembles human calcitonin, but is more active. At present, it is produced either by recombinant DNA technology or by chemical peptide synthesis. The pharmacological properties of the synthetic and recombinant peptides have been demonstrated to be qualitatively and quantitatively equivalent.
- “Electronic Medicines Compendium”. Archived from the original on 2005-11-08. Retrieved 2008-08-07.
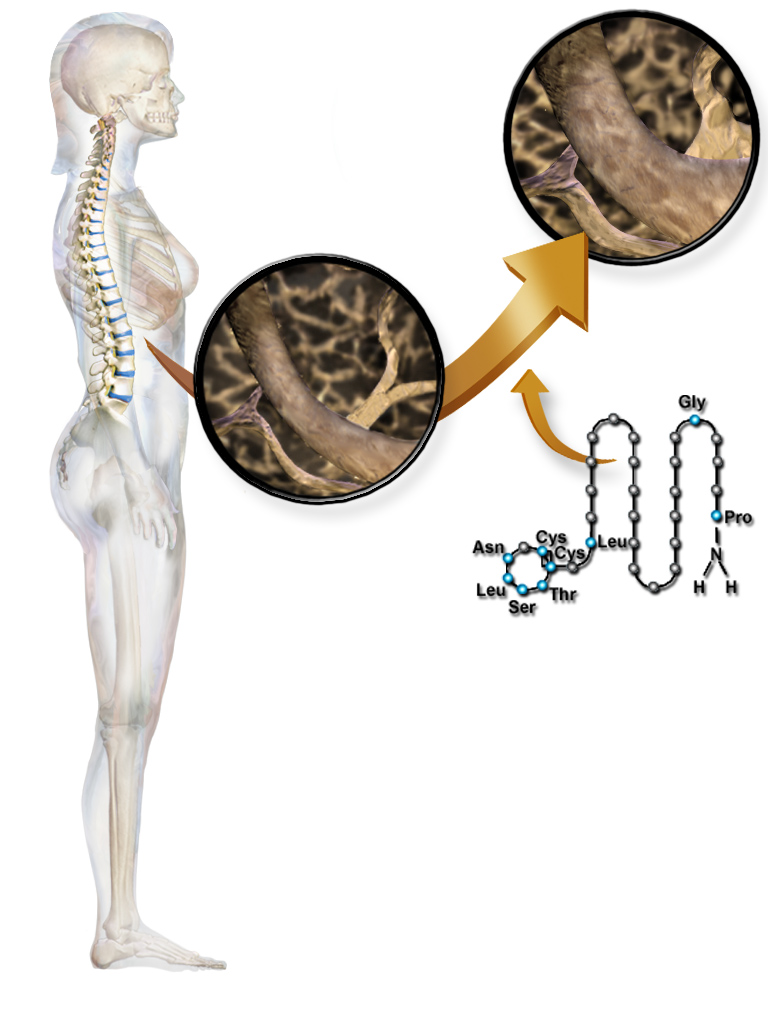
Uses of calcitonin
Treatments
Calcitonin can be used therapeutically for the treatment of hypercalcemia or osteoporosis.
- Inzerillo AM, Zaidi M, Huang CL (2004). “Calcitonin: physiological actions and clinical applications”. Journal of Pediatric Endocrinology & Metabolism. 17 (7): 931–940. doi:10.1007/s00198-015-3149-3. PMID 15301040. S2CID 23551343.
In a recent clinical study, subcutaneous injections of calcitonin have reduced the incidence of fractures and reduced the decrease in bone mass in women with type 2 diabetes complicated with osteoporosis.
- Dexue L, Yueyue Z (November 2014). “Salmon calcitonin in the treatment of elderly women with type 2 diabetes complicated with osteoporosis”. Pak J Pharm Sci. 27 (6 Suppl): 2079–2081. PMID 25410076.
Subcutaneous injections of calcitonin in patients with mania resulted in significant decreases in irritability, euphoria and hyperactivity and hence calcitonin holds promise for treating bipolar disorder. However no further work on this potential application of calcitonin has been reported.
- Vik A, Yatham LN (March 1998). “Calcitonin and bipolar disorder: a hypothesis revisited”. Journal of Psychiatry & Neuroscience. 23 (2): 109–117. PMC 1188909. PMID 9549251.
Diagnostics
It may be used diagnostically as a tumor marker for medullary thyroid cancer, in which high calcitonin levels may be present and elevated levels after surgery may indicate recurrence. It may even be used on biopsy samples from suspicious lesions (e.g., lymph nodes that are swollen) to establish whether they are metastases of the original cancer.
Cutoffs for calcitonin to distinguish cases with medullary thyroid cancer have been suggested to be as follows, with a higher value increasing the suspicion of medullary thyroid cancer:
- Basuyau JP, Mallet E, Leroy M, Brunelle P (October 2004). “Reference intervals for serum calcitonin in men, women, and children”. Clin. Chem. 50 (10): 1828–1830. doi:10.1373/clinchem.2003.026963. PMID 15388660.
- females: 5 ng/L or pg/mL
- males: 12 ng/L or pg/mL
- children under 6 months of age: 40 ng/L or pg/mL
- children between 6 months and 3 years of age: 15 ng/L or pg/mL
- When over 3 years of age, adult cutoffs may be used
Increased levels of calcitonin have also been reported for various other conditions. They include: C-cell hyperplasia, nonthyroidal oat cell carcinoma, nonthyroidal carcinoma and other nonthyroidal malignancies, acute kidney injury and chronic kidney failure, hypercalcemia, hypergastrinemia, and other gastrointestinal disorders, and pulmonary disease.
- Burtis CA, Ashwood ER, Bruns DE (2012). Tietz Textbook of Clinical Chemistry and Molecular Diagnostics (5th ed.). Elsevier Saunders. p. 1774. ISBN 978-1-4160-6164-9.
Structure
Calcitonin is a polypeptide hormone of 32 amino acids, with a molecular weight of 3454.93 daltons. Its structure comprises a single alpha helix.
- Andreotti G, Méndez BL, Amodeo P, Morelli MA, Nakamuta H, Motta A (August 2006). “Structural determinants of salmon calcitonin bioactivity: the role of the Leu-based amphipathic alpha-helix”. J. Biol. Chem. 281 (34): 24193–241203. doi:10.1074/jbc.M603528200. PMID 16766525.
Alternative splicing of the gene coding for calcitonin produces a distantly related peptide of 37 amino acids, called calcitonin gene-related peptide (CGRP), beta type.
- “calcitonin domain annotation”. SMART (a Simple Modular Architecture Research Tool). embl-heidelberg.de. Retrieved 2009-02-22.
The following are the amino acid sequences of salmon and human calcitonin:[citation needed]
- salmon:
Cys-Ser-Asn-Leu-Ser-Thr-Cys-Val-Leu-Gly-Lys-Leu-Ser-Gln-Glu-Leu-His-Lys-Leu-Gln-Thr-Tyr-Pro-Arg-Thr-Asn-Thr-Gly-Ser-Gly-Thr-Pro
- human:
Cys-Gly-Asn-Leu-Ser-Thr-Cys-Met-Leu-Gly-Thr-Tyr-Thr-Gln-Asp-Phe-Asn-Lys-Phe-His-Thr-Phe-Pro-Gln-Thr-Ala-Ile-Gly-Val-Gly-Ala-Pro
Compared to salmon calcitonin, human calcitonin differs at 16 residues.
- “Salmon calicitonin”. prospecbio.
Research
In addition to the injectable and nasal spray dosage forms of the salmon calcitonin, noninvasive oral formulations of the peptide are currently under clinical development. The short-half-life of this peptide in serum triggered several attempts to enhance plasma concentrations. The peptide is complexed with a macromolecule that acts as an absorption enhancer through the transcellular pathway and, additionally, protects the peptide from the harsh pH and enzymatic conditions of the GI tract. This complexation is weak, noncovalent and reversible and the drug remains chemically unmodified. After passage through the intestine, the delivery agent dissociates from the peptide. One of the extensively studied oral formulations is the disodium salts of 5-CNAC oral calcitonin. This novel oral platform in a number of clinical trials at different phases has demonstrated promising enhanced pharmacokinetic profile, high bioavailability, well-established safety and comparable efficacy to that of nasal calcitonin especially for treatment of postmenopausal bone loss.
- das Neves, José; Sarmento, Bruno (2014). Mucosal Delivery of Biopharmaceuticals. Boston: Springer US. pp. 407–422. doi:10.1007/978-1-4614-9524-6_18. ISBN 978-1461495246.
See also
- Procalcitonin
- Procalcitonin (PCT) is a peptide precursor of the hormone calcitonin, the latter being involved with calcium homeostasis. It arises once preprocalcitonin is cleaved by ENDOPEPTIDASE. It was first identified by Leonard J. Deftos and Bernard A. Roos in the 1970s. It is composed of 116 amino acids and is produced by parafollicular cells (C cells) of the thyroid and by the neuroendocrine cells of the lung and the intestine.
- “Procalcitonin: Reference Range, Interpretation, Collection and Panels”. 2017-03-09.
- Deftos LJ, Roos BA, Parthemore JG (December 1975). “Calcium and skeletal metabolism”. The Western Journal of Medicine. 123 (6): 447–58. PMC 1130411. PMID 1105981.
- The level of procalcitonin in the blood stream of healthy individuals is below the limit of detection (0.01 µg/L) of clinical assays. The level of procalcitonin rises in a response to a pro-inflammatory stimulus, especially of bacterial origin. It is therefore often classed as an acute phase reactant. The induction period for procalcitonin ranges from 4–12 hours with a half-life spanning anywhere from 22–35 hours. It does not rise significantly with viral or non-infectious inflammations. In the case of viral infections this is due to the fact that one of the cellular responses to a viral infection is to produce interferon gamma, which also inhibits the initial formation of procalcitonin. With the inflammatory cascade and systemic response that a severe infection brings, the blood levels of procalcitonin may rise multiple orders of magnitude with higher values correlating with more severe disease. However, the high procalcitonin levels produced during infections are not followed by a parallel increase in calcitonin or a decrease in serum calcium levels.
- Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, Bohuon C (December 1994). “Procalcitonin increase after endotoxin injection in normal subjects”. The Journal of Clinical Endocrinology and Metabolism. 79 (6): 1605–8. doi:10.1210/jcem.79.6.7989463. PMID 7989463.
- Long SS, Pickering LK, Prober CG, eds. (2012). “Bacterial infections in the neonate”. Principles and Practice of Pediatric Infectious Diseases (4th ed.). Elsevier. ISBN 978-1437727029.
- Reinhart K, Karzai W, Meisner M (September 2000). “Procalcitonin as a marker of the systemic inflammatory response to infection”. Intensive Care Medicine. 26 (9): 1193–200. doi:10.1007/s001340000624. PMC 7095266. PMID 11089742
- Sandkovsky, Uriel; Kalil, Andre C.; Florescu, Diana F. (2015-06-22). “The use and value of procalcitonin in solid organ transplantation”. Clinical Transplantation. 29 (8): 689–696. doi:10.1111/ctr.12568. ISSN 0902-0063. PMID 25996831. S2CID 10673879.
- Meisner M (2010). Procalcitonin biochemistry and clinical diagnosis (1st ed.). Bremen: UNI-MED-Verl. ISBN 9783837412413. OCLC 697831954.
- Assicot, M.; Bohuon, C.; Gendrel, D.; Raymond, J.; Carsin, H.; Guilbaud, J. (1993-02-27). “High serum procalcitonin concentrations in patients with sepsis and infection”. Lancet. 341 (8844): 515–518. doi:10.1016/0140-6736(93)90277-N. ISSN 0140-6736. PMC 7141580. PMID 8094770.
- Excessive overdose on amphetamine or its analogs can induce systemic inflammation; in a case report of amphetamine overdose, without bacterial infection, significant elevations in procalcitonin were observed.
- Lovas A, Agoston Z, Késmárky K, Hankovszky P, Molnár Z (2014). “Extreme Procalcitonin Elevation without Proven Bacterial Infection Related to Amphetamine Abuse”. Case Reports in Critical Care. 2014: 1–3. doi:10.1155/2014/179313. PMC 4006559. PMID 24826347.
- Glennon RA (2013). “Phenylisopropylamine stimulants: amphetamine-related agents”. In Lemke TL, Williams DA, Roche VF, Zito W (eds.). Foye’s principles of medicinal chemistry (7th ed.). Philadelphia, US: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39). … The phase 1 metabolism of amphetamine analogs is catalyzed by two systems: cytochrome P450 and flavin monooxygenase. … Amphetamine can also undergo aromatic hydroxylation to p-hydroxyamphetamine. … Subsequent oxidation at the benzylic position by DA β-hydroxylase affords p-hydroxynorephedrine. Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). “Biosynthesis of amphetamine analogs in plants”. Trends in Plant Science. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). … Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. … For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. …[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines.
- Procalcitonin (PCT) is a peptide precursor of the hormone calcitonin, the latter being involved with calcium homeostasis. It arises once preprocalcitonin is cleaved by ENDOPEPTIDASE. It was first identified by Leonard J. Deftos and Bernard A. Roos in the 1970s. It is composed of 116 amino acids and is produced by parafollicular cells (C cells) of the thyroid and by the neuroendocrine cells of the lung and the intestine.
Further reading
- MacIntyre I, Alevizaki M, Bevis PJ, Zaidi M (April 1987). “Calcitonin and the peptides from the calcitonin gene”. Clinical Orthopaedics and Related Research. 217 (217): 45–55. doi:10.1097/00003086-198704000-00007. PMID 3549095.
- Di Angelantonio S, Giniatullin R, Costa V, Sokolova E, Nistri A (July 2003). “Modulation of neuronal nicotinic receptor function by the neuropeptides CGRP and substance P on autonomic nerve cells”. British Journal of Pharmacology. 139 (6): 1061–1073. doi:10.1038/sj.bjp.0705337. PMC 1573932. PMID 12871824.
- Findlay DM, Sexton PM (December 2004). “Calcitonin”. Growth Factors. 22 (4): 217–224. doi:10.1080/08977190410001728033. PMID 15621724. S2CID 218910711.
- Sponholz C, Sakr Y, Reinhart K, Brunkhorst F (2007). “Diagnostic value and prognostic implications of serum procalcitonin after cardiac surgery: a systematic review of the literature”. Critical Care. 10 (5): R145. doi:10.1186/cc5067. PMC 1751067. PMID 17038199.
- Schneider HG, Lam QT (August 2007). “Procalcitonin for the clinical laboratory: a review”. Pathology. 39 (4): 383–390. doi:10.1080/00313020701444564. PMID 17676478. S2CID 28018130.
- Grani G, Nesca A, Del Sordo M, Calvanese A, Carbotta G, Bianchini M, Fumarola A (June 2012). “Interpretation of serum calcitonin in patients with chronic autoimmune thyroiditis”. Endocrine-Related Cancer. Bioscientifica. 19 (3): 345–349. doi:10.1530/ERC-12-0013. PMID 22399011.
External links
Wikimedia Commons has media related to Calcitonin.
- The Calcitonin Protein
- Calcitonin at the US National Library of Medicine Medical Subject Headings (MeSH)
- Genes on human chromosome 11
- Peptide hormones
- Hormones of the thyroid gland
- Human hormones
- Hormones of calcium metabolism
- Tumor markers
- Thyroid
From Wikipedia where this page was last updated July 16, 2022


Leave a Reply