Tetralin (1,2,3,4-tetrahydronaphthalene) is a hydrocarbon having the chemical formula C10H12. It is a partially hydrogenated derivative of naphthalene. It is a colorless liquid that is used as a hydrogen-donor solvent.
- Collin, Gerd; Höke, Hartmut; Greim, Helmut (2003). “Naphthalene and Hydronaphthalenes”. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_001.pub2.
Production
Tetralin is produced by the catalytic hydrogenation of naphthalene.
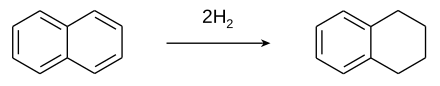
Although nickel catalysts are traditionally employed, many variations have been evaluated. Over-hydrogenation converts tetralin into decahydronaphthalene (decalin). Rarely encountered is dihydronaphthalene (dialin).
- Krichko, A. A.; Skvortsov, D. V.; Titova, T. A.; Filippov, B. S.; Dogadkina, N. E. (1969). “Production of tetralin by the hydrogenation of naphthalene-containing fractions”. Chemistry and Technology of Fuels and Oils. 5: 18–22. doi:10.1007/BF00727949. S2CID 95026822.
Laboratory methods
In a classic named reaction called the Darzens tetralin synthesis, named for Auguste Georges Darzens (1926), derivatives can be prepared by intramolecular electrophilic aromatic substitution reaction of a 1-aryl-4-pentene using concentrated sulfuric acid,
- Michael B. Smith (2011). Organic Synthesis (third ed.). Academic Press. pp. 1209–1210. ISBN 9780124158849.
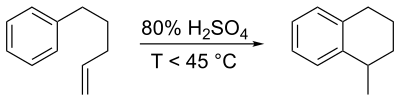
Uses
Tetralin is used as a hydrogen-donor solvent, for example in coal liquifaction. It functions as a source of H2, which is transferred to the coal. The partially hydrogenated coal is more soluble.
- Collin, Gerd; Höke, Hartmut; Greim, Helmut (2003). “Naphthalene and Hydronaphthalenes”. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_001.pub2.
- Isa, Khairuddin Md.; Abdullah, Tuan Amran Tuan; Md. Ali, Umi Fazara (2018). “Hydrogen donor solvents in liquefaction of biomass: A review”. Renewable & Sustainable Energy Reviews. 81(Part_1): 1259-1268. doi:10.1016/j.rser.2017.04.006.
It has been used in sodium-cooled fast reactors as a secondary coolant to keep sodium seals around pump impellers solidified; however its use has been superseded by NaK.
- US Atomic Energy Commission (1961) SRE Core Recovery Remediation method after a failure in the moderator cans due to a crack in the secondary coolant tubes in the SRE, Spring 1959. This caused a leak of Tetralin into the reactor.
It is also used for the laboratory synthesis of HBr:C10H12 + 4 Br2 → C10H8Br4 + 4 HBr
The facility of this reaction is in part a consequence of the moderated strength of the benzylic C-H bonds.
Safety
LD50 (rats, oral) is 2.68 g/kg. Tetralin induces methemoglobinemia.
- Collin, Gerd; Höke, Hartmut; Greim, Helmut (2003). “Naphthalene and Hydronaphthalenes”. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_001.pub2.
References
- Gonçalves, F. A.; Hamano, K.; Sengers, J. V. (1989). “Density and viscosity of tetralin and trans-decalin”. International Journal of Thermophysics. 10 (4): 845. Bibcode:1989IJT….10..845G. doi:10.1007/BF00514480. S2CID 119843498.
- Collin, Gerd; Höke, Hartmut; Greim, Helmut (2003). “Naphthalene and Hydronaphthalenes”. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_001.pub2.
- Krichko, A. A.; Skvortsov, D. V.; Titova, T. A.; Filippov, B. S.; Dogadkina, N. E. (1969). “Production of tetralin by the hydrogenation of naphthalene-containing fractions”. Chemistry and Technology of Fuels and Oils. 5: 18–22. doi:10.1007/BF00727949. S2CID 95026822.
- Michael B. Smith (2011). Organic Synthesis (third ed.). Academic Press. pp. 1209–1210. ISBN 9780124158849.
- Isa, Khairuddin Md.; Abdullah, Tuan Amran Tuan; Md. Ali, Umi Fazara (2018). “Hydrogen donor solvents in liquefaction of biomass: A review”. Renewable & Sustainable Energy Reviews. 81(Part_1): 1259-1268. doi:10.1016/j.rser.2017.04.006.
- US Atomic Energy Commission (1961) SRE Core Recovery Remediation method after a failure in the moderator cans due to a crack in the secondary coolant tubes in the SRE, Spring 1959. This caused a leak of Tetralin into the reactor.
Wikimedia Commons has media related to Tetralins.
Subcategories
- Aminotetralins (2 C, 18 P)
- 1-Aminotetralins (8 P)
Pages in category “Tetralins”
0–9
- 6-APT
- sometimes called tetralinylaminopropane (TAP), is a drug of the amphetamine class which acts as a selective serotonin releasing agent (SSRA). It has IC50 values of 121 nM, 6,436 nM, and 3,371 nM for inhibiting the reuptake of serotonin, dopamine, and norepinephrine, respectively. Though it possesses an appreciable in vitro profile, in animal drug discrimination studies it was not found to substitute for MMAI or amphetamine and to only partially substitute for MBDB. This parallels Alexander Shulgin‘s finding that EDMA (the 1,4-benzodioxine analogue of 6-APT) is inactive, and appears to indicate that the pharmacokinetics of both EDMA and 6-APT may not be favorable. Monte AP, Marona-Lewicka D, Cozzi NV, Nichols DE (November 1993). “Synthesis and pharmacological examination of benzofuran, indan, and tetralin analogues of 3,4-(methylenedioxy)amphetamine”. Journal of Medicinal Chemistry. 36 (23): 3700–6. doi:10.1021/jm00075a027. PMID 8246240.. Ann Shulgin; Alexander Shulgin (1991). Pihkal: A Chemical Love Story. Transform Press. ISBN 0-9630096-0-5.
B
- Belladonnine
- Belladonnine is a member of class of tropane alkaloids. Belladonnine can be found in plants of family Solanaceae. Commercially available preparations called “belladonnine” are sometimes a mixture of this chemical with atropine. Pubchem. “Belladonnine”. nih.gov. Merling, G. (1884). “Ueber Belladonin” (PDF). Chemische Berichte. 17: 381–385. doi:10.1002/cber.188401701108.
- Bexarotene
- Bexarotene, sold under the brand Targretin, is an antineoplastic (anti-cancer) agent used for the treatment of cutaneous T cell lymphoma (CTCL). It is a third-generation retinoid. Bexarotene is indicated for the treatment of cutaneous manifestations of cutaneous T-cell lymphoma in people who are refractory to at least one prior systemic therapy (oral) and for the topical treatment of cutaneous lesions in patients with CTCL who have refractory or persistent disease after other therapies or who have not tolerated other therapies (topical).
- Known contraindications include:
- Hypersensitivity to the active substance or to any of the excipients in the preparation(s).
- Pregnancy and lactation
- Women of child-bearing potential without effective birth-control measures
- History of pancreatitis
- Uncontrolled hypercholesterolaemia
- Uncontrolled hypertriglyceridaemia
- Hypervitaminosis A
- Uncontrolled thyroid disease
- Hepatic insufficiency
- Ongoing systemic infection
- It has been used off-label for non-small cell lung cancer and breast cancer. Overall the most common adverse effects are skin reactions (mostly itchiness and rashes), leucopenia, headache, weakness, thyroid anomalies (which seem to be mediated by RXR-mediated downregulation of thyroid stimulating hormone) and blood lipid anomalies such as hypercholesterolaemia (high blood cholesterol) and hyperlipidaemia, hypothyroidism. “TARGRETIN (BEXAROTENE) CAPSULE [CARDINAL HEALTH]”. DailyMed. Cardinal Health. March 2006. Retrieved 12 January 2014. Esteva FJ, Glaspy J, Baidas S, Laufman L, Hutchins L, Dickler M, et al. (March 2003). “Multicenter phase II study of oral bexarotene for patients with metastatic breast cancer” (PDF). Journal of Clinical Oncology. 21 (6): 999–1006. doi:10.1200/JCO.2003.05.068. PMID 12637463. “Targretin Capsules – Summary of Product Characteristics”. electronic Medicines Compendium. Eisai Ltd. 4 April 2013. Retrieved 14 January 2014. Brunton L, Chabner B, Knollman B (2010). Goodman and Gilman’s The Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill Professional. ISBN 978-0-07-162442-8. “Targretin (bexarotene) dosing, indications, interactions, adverse effects, and more”. Medscape Reference. WebMD. Retrieved 31 January 2014. Dragnev KH, Petty WJ, Shah SJ, Lewis LD, Black CC, Memoli V, et al. (March 2007). “A proof-of-principle clinical trial of bexarotene in patients with non-small cell lung cancer” (PDF). Clinical Cancer Research. 13 (6): 1794–800. doi:10.1158/1078-0432.CCR-06-1836. PMID 17363535. S2CID 25374661.
- Brodifacoum
- Brodifacoum is a highly lethal 4-hydroxycoumarin vitamin K antagonist anticoagulant poison. In recent years, it has become one of the world’s most widely used pesticides. It is typically used as a rodenticide, but is also used to control larger pests such as possum. Brodifacoum has an especially long half-life in the body, which ranges up to nine months, requiring prolonged treatment with antidotal vitamin K for both human and pet poisonings. It has one of the highest risks of secondary poisoning to both mammals and birds. Significant experience in brodifacoum poisonings has been gained in many human cases where it has been used in attempted suicides, necessitating long periods of vitamin K treatment. In March 2018, cases of severe coagulopathy and bleeding associated with synthetic cannabinoid use contaminated with brodifacoum were reported in five states of the US. Eason, C.T. and Wickstrom, M. Vertebrate pesticide toxicology manual, New Zealand Department of Conservation Rodenticides: Topic Fact Sheet, National Pesticide Information Center “3 arrested in Chicago in connection to synthetic pot; 2 deaths, 54 others cases of severe bleeding”. WGN-TV. 2 April 2018.
- Butidrine
- Butidrine (INN), or butedrine or butydrine, also known as hydrobutamine or idrobutamine, is a beta blocker related to pronethalol and propranolol that was developed in the 1960s. Similarly to certain other beta blockers, butidrine also possesses local anesthetic properties. Bristol JA (1986). Cardiovascular drugs. John Wiley & Sons, Incorporated. p. 111. ISBN 978-0-471-09228-5. Drug Metabolism Reviews. Marcel Dekker. 1972. Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 58–. ISBN 978-94-011-4439-1. Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 197–. ISBN 978-1-4757-2085-3. Charlier R (1971). Antianginal drugs: pathophysiological, haemodynamic, methodological, pharmacological, biochemical and clinical basis for their use in human therapeutics. Springer-Verlag. ISBN 978-3-540-05365-1.
C
- Coumatetralyl
- Coumatetralyl is an anticoagulant of the 4-hydroxycoumarin vitamin K antagonist type used as a rodenticide. Coumatetralyl is commonly used with grains and other cereals as a rodent poison in conjunction with a tracking powder to monitor feeding activity in a particular area. Tracking powder also clings to fur, which allows more poison to be ingested from grooming. Concentrations of the chemical are usually 500 mg per 1 kg of bait. Symptoms of overexposure relate to failure of the blood clotting mechanism and include bleeding gums and failure of blood clotting after skin wounds. After one exposure the toxicity of coumatetralyl is relatively low; however, if overexposure continues for several days the product becomes more toxic. The product must therefore be constantly present in the bloodstream for more than one to two days in order to be highly toxic. A single exposure, even though relatively large, may not produce toxic symptoms as the compound is quite rapidly metabolized. Vitamin K1 (phylloquinone) is antidotal. “Coumatetralyl”. PubChem. National Center for Biotechnology Information. Retrieved 2021-11-25. J. Routt Reigart and James R. Roberts, ed. (2013). “Chapter 18: Rodenticides”. Recognition and Management of Pesticide Poisonings (PDF) (6th ed.).
D
- Difenacoum
- Difenacoum is an anticoagulant of the 4-hydroxycoumarin vitamin K antagonist type. It has anticoagulant effects and is used commercially as a rodenticide. It was first introduced in 1976 and first registered in the USA in 2007 as a rodenticide effective against rats and mice which were resistant to other anticoagulants. Because other species of mammals and birds may prey upon affected rodents, or directly ingest rodenticide bait, there is a risk of primary, secondary or tertiary exposure; examples are described in a 2012 publication on veterinary toxicology. Using radiolabeled isotopes, difenacoum (and/or its metabolites) has been shown to be distributed across many organ tissues upon oral ingestion, with the highest concentrations occurring in the liver and pancreas.Difenacoum has been shown to be highly toxic to some species of freshwater fish and green algae despite the fact that difenacoum is weakly soluble in aqueous solutions. “University of Hertfordshire: IUPAC: difenacoum”. Retrieved 3 April 2015. Gupta, Ramesh C. (ed) (2012). Veterinary Toxicology: Basic and clinical principles. Academic Press. p. 673-697. ISBN 9780123859273. Retrieved 3 April 2015.
- Difethialone
- Difethialone is an anticoagulant used as a rodenticide. It is considered a second generation agent.In May 2008, the EPA added restrictions on the sale of difethialone in consumer-use rodenticide products and also for exterior use by commercial applicators Nahas K, Lorgue G, Mazallon M (1989). “Difethialone (LM-2219): a new anticoagulant rodenticide for use against warfarin-resistant and -susceptible strains of Rattus norvegicus and Mus musculus”. Annales de Recherches Vétérinaires. 20 (2): 159–64. PMID 2751229. Saravanan K, Kanakasabai R, Thiyagesan K (June 2003). “Field evaluation of difethialone, a new second generation anticoagulant rodenticide in the rice fields”. Indian Journal of Experimental Biology. 41 (6): 655–8. PMID 15266918. EPA, OCSPP, OPP, US (2014-03-04). “Restrictions on Rodenticide Products”. www.epa.gov. “Regulations.gov”. www.regulations.gov.
E
- Elacestrant
- Elacestrant (INN) is a nonsteroidal combined selective estrogen receptor modulator (SERM) and selective estrogen receptor degrader (SERD).
F
- Flocoumafen
- Flocoumafen is a fluorinated, second-generation anticoagulant of the 4-hydroxycoumarin vitamin K antagonist type. It is a second generation chemical in this class, used commercially as a rodenticide. It has a very high toxicity and is restricted to indoor use and sewers. This restriction is mainly due to the increased risk to non-target species, especially due to its tendency to bio-accumulate in exposed organisms. Studies have shown that rodents resistant to first-generation anticoagulants can be adequately controlled with flocoumafen. It was synthesized in 1984 by Shell International Chemical. Flocoumafen — A new anticoagulant rodenticide
L
- Lasofoxifene
- Lasofoxifene, sold under the brand name Fablyn, is a nonsteroidal selective estrogen receptor modulator (SERM) which is marketed by Pfizer in Lithuania and Portugal for the prevention and treatment of osteoporosis and for the treatment of vaginal atrophy
- Liranaftate
- Liranaftate (trade name Zefnart) is a topical antifungal drug. It is used as a 2% cream used to treat tinea pedis (athlete’s foot), tinea corporis (ringworm), and tinea cruris (jock itch). It was approved for use in Japan in August 2000. Liranaftate works by inhibiting the fungal enzyme squalene epoxidase that is necessary for the fungus to synthesize sterols which are essential for cell membrane integrity. Koga H, Nanjoh Y, Makimura K, Tsuboi R (2009). “In vitro antifungal activities of luliconazole, a new topical imidazole”. Medical Mycology. 47 (6): 640–7. doi:10.1080/13693780802541518. PMID 19115136. “Torii Pharmaceutical to Launch Antifungal Agent for External Use, “ZEFNART SOLUTION 2%”, in Japan” (Press release). Torii Pharmaceutical Co. Retrieved June 27, 2021. “Liranaftate”. ncats.io. Retrieved June 27, 2021. “Liranaftate”. Adis Insight. Retrieved June 27, 2021. “Liranaftate”. targetmol.com. Retrieved June 27, 2021.
M
- Mibefradil
- Mibefradil was a pharmaceutical drug used for the treatment of hypertension and chronic angina pectoris. It is a nonselective calcium channel blocker. It was voluntary pulled from the market ten months after FDA approval, citing potential serious health hazards shown in post release studies. “The withdrawal came after reports of dangerous and even fatal interactions with at least 25 other drugs, including common antibiotics, antihistamines, and cancer drugs…Posicor inhibits cytochrome P450 2D6 and 3A4 and could interact by increasing the plasma concentrations of concomitantly administered drugs. At times, the FDA wrote, this increased accumulation of drugs reached dangerous levels in the body. Interactions, particularly with those drugs that also affect liver metabolism, appeared to cause catabolism of important abdominal muscles in a few people. The interaction was considered extremely dangerous…In an unusual move, the Journal of the American Medical Association allowed the premature release of information scheduled for its July 8, 1998, issue about the dangers posed by switching patients from Posicor to other calcium channel blockers such as felodipine and timolol. In his report, Michael E. Mullins, MD, of the Oregon Health Sciences University outlined the cases of 4 patients who had gone into shock within 12 hours of the drug switch…Mullins said it can take many days for Posicor to leave the patient’s system, and he recommended a 7-day period between ending Posicor and beginning other calcium channel blockers or β-blockers. Felodipine and timolol require a 14-day waiting period.” Ruth SoRelle, Withdrawal of Posicor From Market, AHA Journal – Circulation, Vol 98, Issue 9, September 1, 1998 doi.org/10.1161/01.CIR.98.9.831
N
- Nadolol
- Nadolol, sold under the brand name Corgard among others, is a medication used to treat high blood pressure, heart pain, atrial fibrillation, and some inherited arrhythmic syndromes. It has also been used to prevent migraine headaches and complications of cirrhosis. Common side effects include dizziness, feeling tired, a slow heart rate, and Raynaud syndrome. Serious side effects may include heart failure and bronchospasm. Its use in pregnancy and breastfeeding is of unclear safety. It is a non-selective beta blocker and works by blocking β1-adrenergic receptors in the heart and β2-adrenergic receptors in blood vessels.Nadolol was patented in 1970 and came into medical use in 1978. It is available as a generic medication. In 2020, it was the 340th most commonly prescribed medication in the United States, with more than 700 thousand prescriptions. “Nadolol Monograph for Professionals”. Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019. “Nadolol Pregnancy and Breastfeeding Warnings”. Drugs.com. Retrieved 3 March 2019. Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 460. ISBN 9783527607495. “Nadolol – Drug Usage Statistics”. ClinCalc. Retrieved 7 October 2022.
- Nafenopin
- Nafenopin is a hypolipidemic agent. Levine, W.G.; Meijer, D.K.F. (1977), “THE CHOLERETIC EFFECT OF NAFENOPIN”, Abstracts, Elsevier, p. 395, retrieved 2022-12-21
- Naflocort
- Naflocort is a synthetic glucocorticoid corticosteroid which was never marketed. (That’s it? No other information? That can’t be good. What happened to those who took it? Did they spontaneously combust? Or what?) Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 848–. ISBN 978-1-4757-2085-3.
- Nepinalone
- Nepinalone is a cough suppressant. Its brand names include Placatus, Tussolvina, and Nepitus. The effect is evident after 20–30 minutes after administration and persists for at least 4 hours. It acts primarily at the level of the CNS, but also shows a slight activity in inhibiting the bronchospasm. In such use, it is less effective than codeine and more effective than dextromethorphan in inhibiting the tussive stimulus. The starting material is called 2-Phenylpropanoyl Chloride [22414-26-2]. This is reacted with ethylene in the presence of aluminum trichloride catalyst to give 1-methyl-2-tetralone [66405-14-9] (1). Base catalyzed alkylation of this with 1-(2-chloroethyl)piperidine [1932-03-2] (2) gives Nepinalone (3). GB1140990 idem Raffaello Fusco & Franco Tenconi, U.S. Patent 3,576,811 (1971 to Warner Lambert Co LLC). Franco Tenconi, Silvana Furfaro, EP 0507001 (1992 to BIOINDUSTRIA FARMACEUTICI S.p.A.).
- Nirogacestat
- Nirogacestat (PF-03084014) is a selective gamma secretase inhibitor developed by SpringWorks Therapeutics that has potential anti-tumor activity. It was granted FDA breakthrough drug designation in September 2019 for adult patients with progressive, unresectable, recurrent or refractory desmoid tumors or deep fibromatosis. Nirogacestat is currently in Phase 2 clinical trials for unresectable desmoid tumors. In addition, a Phase 3 clinical trial, DeFi, is currently in progress for nirogacestat for adults with desmoid tumors and aggressive fibromatosis. In addition, three trials are presently recruiting patients that include nirogacestat with other anticancer therapies in multiple myeloma, including the UNIVERSAL study for nirogacestat with the allogeneic CAR-T therapy ALLO-715. Chen X, Chen X, Zhou Z, Mao Y, Wang Y, Ma Z, Xu W, Qin A, Zhang S (September 2019). “Nirogacestat suppresses RANKL-Induced osteoclast formation in vitro and attenuates LPS-Induced bone resorption in vivo”. Experimental Cell Research. 382 (1): 111470. doi:10.1016/j.yexcr.2019.06.015. PMID 31211955. S2CID 195065514. “FDA Grants Nirogacestat Breakthrough Designation for Desmoid Tumors”. OncLive. Retrieved 2021-06-25. Clinical trial number NCT04195399 for “A Safety, Pharmacokinetic and Efficacy Study of a y-Secretase Inhibitor, Nirogacestat (PF-03084014) in Children and Adolescents With Progressive, Surgically Unresectable Desmoid Tumors” at ClinicalTrials.gov. linical trial number NCT03785964 for “A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial of Nirogacestat Versus Placebo in Adult Patients With Progressing Desmoid Tumors/Aggressive Fibromatosis (DT/AF)” at ClinicalTrials.gov. Clinical trial number NCT04093596 for “A Single-Arm, Open-Label, Phase 1 Study of the Safety, Efficacy, and Cellular Kinetics/Pharmacodynamics of ALLO-715 to Evaluate an Anti-BCMA Allogeneic CAR T Cell Therapy With or Without Nirogacestat in Subjects With Relapsed/Refractory Multiple Myeloma” at ClinicalTrials.gov. Clinical trial number NCT04722146 for “A Multi-arm Phase 1b Study of Teclistamab With Other Anticancer Therapies in Participants With Multiple Myeloma” at ClinicalTrials.gov. Clinical trial number NCT04126200 for “A Phase I/II, Randomized, Open-label Platform Study Utilizing a Master Protocol to Study Belantamab Mafodotin (GSK2857916) as Monotherapy and in Combination With Anti-Cancer Treatments in Participants With Relapsed/Refractory Multiple Myeloma (RRMM) – DREAMM 5” at ClinicalTrials.gov
P
- Palovarotene
- Palovarotene, sold under the brand name Sohonos, is a medication used for the treatment of heterotopic ossification (HO) and fibrodysplasia ossificans progressiva (FOP). It is a highly selective retinoic acid receptor gamma (RARγ) agonist. Palovarotene is being developed by Ipsen Biopharmaceuticals and was granted Fast Track and orphan drug designations by the United States Food and Drug Administration for the treatment of FOP and Orphan Medicinal Product Designation by the European Medicines Agency (EMA) in 2014. Phase II clinical studies failed to show a significant change in heterotopic bone volume, the main outcome measure, but prompted further investigation in a Phase III clinical trial. In December 2019, Ipsen issued a partial clinical hold for people under the age of 14, due to reports of early fusion of growth plates. Ipsen acquired Clementia in 2019. “FOP Fact Sheets”. www.ifopa.org. Archived from the original on 4 April 2016. Retrieved 11 April 2016. “Health Canada Approves Ipsen’s Sohonos (palovarotene capsules) as the First Approved Treatment for Fibrodysplasia Ossificans Progressiva” (Press release). Ipsen. 24 January 2022. Retrieved 28 May 2022 – via Business Wire. “Public summary of opinion on orphan designation. Palovarotene for the treatment of fibrodysplasia ossificans progressiva” (PDF). www.ema.europa.eu. Committee for Orphan Medicinal Products. Retrieved 11 April 2016. “Clementia Pharmaceuticals Receives Fast Track Designation for Palovarotene for Treatment of Fibrodysplasia Ossificans Progressiva (FOP)”. PRNewswise. 1 December 2014. Retrieved 11 April 2016. “Clementia Pharmaceuticals Receives EMA Orphan Medicinal Product Designation for Palovarotene for the Treatment of Fibrodysplasia Ossificans Progressiva”. PRNewswire. 21 November 2014. Retrieved 11 April 2016. Pignolo, Robert J.; Baujat, Geneviève; Hsiao, Edward C.; Keen, Richard; Wilson, Amy; Packman, Jeff; Strahs, Andrew L.; Grogan, Donna R.; Kaplan, Frederick S. (October 2022). “Palovarotene for Fibrodysplasia Ossificans Progressiva ( FOP ): Results of a Randomized, Placebo‐Controlled, Double‐Blind Phase 2 Trial”. Journal of Bone and Mineral Research. 37 (10): 1891–1902. doi:10.1002/jbmr.4655. ISSN 0884-0431. PMID 35854638. S2CID 250697248. “Ipsen Initiates Partial Clinical Hold for Palovarotene IND120181 and IND135403 Studies”. “Ipsen Completes Acquisition of Clementia Pharmaceuticals”.
R
- RAC 421-II
- RAC 421-II, also referred to simply as RAC 421, is a quaternary local anesthetic that acts through intracellular blockage of the NaKATPase channel. As a quaternary ammonium analogue of another local anesthetic, RAC 109, RAC 421-II is permanently charged and so cannot cross the hydrophobic phospholipid cell membrane. As it cannot diffuse across the cell membrane, it cannot exert its inhibitory effects on the intracellular surface of NaKATPase. As such, it can only exert its anesthetic properties if it is injected into the cytosol of the nerve fibre. Inhibition occurs through allowing the sodium and potassium gradients across the cell membrane to dissipate. NaKATPase blockage preferentially inhibits firing of nociceptive nerve fibres due to their relatively low cell diameter and so low tolerance to NaKATPase inhibitors. This is in contrast to non-quaternary anesthetics like benzocaine and tetracaine which cross the cell membrane in their uncharged states and so they can induce anesthetic effects upon application to the extracellular side of the membrane. They subsequently become charged and so activated within the cytosol to exert their inhibitory effects on NaKATPase (NaKATPase inhibiting anesthetics must be in their charged state to become active). Kwon YW, Triggle DJ (1991). “Chiral aspects of drug action at ion channels: a commentary on the stereoselectivity of drug actions at voltage-gated ion channels with particular reference to verapamil actions at the Ca2+ channel”. Chirality. 3 (5): 393–404. doi:10.1002/chir.530030504. PMID 1721828.
T
- Tamibarotene
- Tamibarotene, also called retinobenzoic acid, is orally active, synthetic retinoid, developed to overcome all-trans retinoic acid (ATRA) resistance, with potential antineoplastic activity against acute promyelocytic leukaemia (APL). Tamibarotene (brand name: Amnolake), also called retinobenzoic acid, is orally active, synthetic retinoid, developed to overcome all-trans retinoic acid (ATRA) resistance, with potential antineoplastic activity against acute promyelocytic leukaemia (APL) . It is currently marketed only in Japan and early trials have demonstrated that it tends to be better tolerated than ATRA. It has also been investigated as a possible treatment for Alzheimer’s disease, multiple myeloma and Crohn’s disease. Tamibarotene is found to have inhibitory potential for the viral spike protein of Omicron variant of SARS-CoV2 and can be a potential candidate for developing potential therapeutics for the treatment of omicron variant of COVID19. “Tamibarotene: AM 80, retinobenzoic acid, Tamibaro”. Drugs in R&D. 5 (6): 359–62. 2004. doi:10.2165/00126839-200405060-00010. PMID 15563242. Miwako, I; Kagechika, H (August 2007). “Tamibarotene”. Drugs of Today. 43 (8): 563–568. doi:10.1358/dot.2007.43.8.1072615. PMID 17925887. Fukasawa, H; Nakagomi, M; Yamagata, N; Katsuki, H; Kawahara, K; Kitaoka, K; Miki, T; Shudo, K (2012). “Tamibarotene: a candidate retinoid drug for Alzheimer’s disease” (PDF). Biological & Pharmaceutical Bulletin. 35 (8): 1206–1212. doi:10.1248/bpb.b12-00314. PMID 22863914. Mujwar S. Computational repurposing of tamibarotene against triple mutant variant of SARS-CoV-2. Comput Biol Med. 2021 Sep;136:104748. doi: 10.1016/j.compbiomed.2021.104748. Epub 2021 Aug 8. PMID: 34388463; PMCID: PMC8349365.
- Tetryzoline
- Tetryzoline is a drug used in some over-the-counter eye drops and nasal sprays. Tetryzoline was patented in 1954, and came into medical use in 1959. Tetryzoline eye drops may cause blurred vision, eye irritation and dilated pupils. Tetryzoline is not suitable for prolonged use as its vasoconstrictive effects within the eye eventually decrease or stop. If tolerance to the drug has developed, ceasing its use may cause a rebound effect and increase redness of the eyes — a vasodilatory effect Intranasal use of tetryzoline may cause transient burning, stinging, or dryness of the mucosa and sneezing. Prolonged intranasal use often causes opposite effects in the form of rebound congestion with effects such as chronic redness, swelling and rhinitis. Prolonged use thus may result in overuse of the drug. In children, it might cause profound sedation. Overdose most often causes slow heart rate. Respiratory depression, low blood pressure, constricted pupils, hypothermia, brief episodes of high blood pressure, drowsiness, headaches and vomiting may also occur. In serious cases some of these effects may result in circulatory shock. Most often overdoses occur in children who have ingested the drug. There is no antidote for tetryzoline or other similar imidazoline analogue poisoning, but the symptoms can be alleviated and with treatment, death is rare. An urban legend suggests that tetryzoline can cause violent diarrhea if given orally, such as by putting a few drops of Visine in an unsuspecting person’s beverage. However, the actual results of the prank may be worse, varying from severe nausea and vomiting to seizures or a coma. Larger doses can cause death. Diarrhea is not a side effect. (and that is no doubt only one instance where the truth is much worse than the urban legend) In late August 2018, a South Carolina woman was charged with murdering her husband by putting eye drops containing tetryzoline in his drinking water. An autopsy found high concentrations of tetryzoline in his body. Tetryzoline has been used as a date rape drug in a number of cases due to its ability to cause dizziness and unconsciousness. In 2018 an elderly woman in Pewaukee, Wisconsin died in an apparent overdose or suicide, however in June 2021 police charged her caregiver with murder, alleging that the death was caused by a water bottle laced with Visine. In 2019, a North Carolina paramedic was accused of using Tetryzoline eye drops to cause the death of his wife. The blood sample results showed about 30 – 40 times higher than the therapeutic level of Tetryzoline. Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 552. ISBN 978-3-527-60749-5. “Tetryzoline”. go.drugbank.com. Retrieved 2022-09-15 Al-Abri SA, Yang HS, Olson KR (December 2014). “Unintentional pediatric ophthalmic tetrahydrozoline ingestion: case files of the medical toxicology fellowship at the University of California, San Francisco”. Journal of Medical Toxicology. 10 (4): 388–91. doi:10.1007/s13181-014-0400-9. PMC 4252297. PMID 24760708. Stillwell ME, Saady JJ (September 2012). “Use of tetrahydrozoline for chemical submission”. Forensic Science International. 221 (1–3): e12-6. doi:10.1016/j.forsciint.2012.04.004. PMID 22554870. Carr ME, Engebretsen KM, Ho B, Anderson CP (November 2011). “Tetrahydrozoline (Visine®) concentrations in serum and urine during therapeutic ocular dosing: a necessary first step in determining an overdose”. Clinical Toxicology. 49 (9): 810–4. doi:10.3109/15563650.2011.615064. PMID 21972870. S2CID 20238499. “Tetryzoline”. go.drugbank.com. Retrieved 2022-12-21. Castillo M, Scott NW, Mustafa MZ, Mustafa MS, Azuara-Blanco A (June 2015). “Topical antihistamines and mast cell stabilisers for treating seasonal and perennial allergic conjunctivitis” (PDF). The Cochrane Database of Systematic Reviews. 6 (6): CD009566. doi:10.1002/14651858.CD009566.pub2. hdl:2164/6048. PMID 26028608. “International Non-Proprietary Names for Pharmaceutical Preparations. Recommended International Non-Proprietary Names: List 3” (PDF). World Health Organization. p. 474. Archived (PDF) from the original on 2016-09-11. Retrieved 30 August 2016. “Visine Prank: Mickey Red Eyes”. Snopes. 29 June 2009. Retrieved 28 July 2010. “US wife accused of ‘fatally poisoning husband with eyedrops'”. BBC. 4 September 2018. Retrieved 4 September 2018. Police: Woman kills husband by putting eye drops in water, Associated Press, Aug 31, 2018 Connelly E (1 September 2018). “Wife admits fatally poisoning ‘unfaithful’ hubby with eye drops: cops” (Newspaper). New York Post. Archived from the original on 2018-09-02. Retrieved 3 September 2018. Hutchinson B (8 June 2021). “Wisconsin woman arrested, accused of murdering friend with eye drops: An investigation alleges the victim’s water bottle was laced with Visine”. ABC News. Jacobo J, Stein B (23 December 2019). “Paramedic accused of fatally poisoning his wife with ingredient found in eye drops: Prosecutors”. ABC News. Retrieved 2022-09-15. Lee BY. “How Visine Eye Drops In The Mouth Can Kill, Here Are Two Cases”. Forbes. Retrieved 2022-09-15. “Tetrahydrozoline”. toxnet.nlm.nih.gov. Archived from the original on 2017-01-03. Retrieved 2018-09-05. McLaurin E, Cavet ME, Gomes PJ, Ciolino JB (March 2018). “Brimonidine Ophthalmic Solution 0.025% for Reduction of Ocular Redness: A Randomized Clinical Trial”. Optometry and Vision Science. 95 (3): 264–271. doi:10.1097/OPX.0000000000001182. PMC 5839712. PMID 29461408. US 2731471, Synerholm M, Jules LH, Sahyun M, “Imidazoline Derivatives”, issued 17 January 1956, assigned to Sahyun Laboratories. Budavari S, O’Neil M, Smith A, Heckelman P, Obenchain J (2000). The Merck Index (12th ed.). Whitehouse Station, NJ, United States: Chapman & Hall Electronic Publishing Division. p. 1453. ISBN 978-1-58488-129-2. OCLC 46987702.
- Tolciclate
- Tolciclate (INN) is an antifungal medication. See Tolnaftate “International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary names: List 15” (PDF). World Health Organization. 1975. Retrieved 12 November 2016. Ryder NS, Frank I, Dupont MC (May 1986). “Ergosterol biosynthesis inhibition by the thiocarbamate antifungal agents tolnaftate and tolciclate”. Antimicrobial Agents and Chemotherapy. 29 (5): 858–60. doi:10.1128/aac.29.5.858. PMC 284167. PMID 3524433. Bianchi A, Monti G, de Carneri I (September 1977). “Tolciclate: further antimycotic studies”. Antimicrobial Agents and Chemotherapy. 12 (3): 429–30. doi:10.1128/aac.12.3.429. PMC 429931. PMID 907333.
- Tramazoline
- Tramazoline is a chemical that is used in the form of tramazoline hydrochloride in nasal decongestant preparations. It is an α-adrenergic receptor agonist that inhibits secretion of nasal mucus. It was patented in 1961 and came into medical use in 1962. “Spray-Tish Consumer Information”. MyDr.com.au. Retrieved 16 April 2011. Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 552. ISBN 9783527607495. “Consumer medicine information: Spray-Tish”. nps.org.au. NPS MedicineWise. Retrieved September 13, 2021.

Leave a Reply