Due to its critical role in regulation of cell proliferation, growth, migration and apoptosis, it is considered to be an oncogene, or tumor suppressor. Contrarily To date, increased CBP activity has been implicated in a variety of different malignancies including breast cancer, lung cancer, prostate cancer, colorectal cancer, acute leukemias, head and neck cancer, and many others.
- Dutta R, Tiu B, Sakamoto KM (September 2016). “CBP/p300 acetyltransferase activity in hematologic malignancies”. Molecular Genetics and Metabolism. 119 (1–2): 37–43. doi:10.1016/j.ymgme.2016.06.013. PMID 27380996.
- Bordonaro M, Lazarova DL (July 2015). “CREB-binding protein, p300, butyrate, and Wnt signaling in colorectal cancer”. World Journal of Gastroenterology. 21 (27): 8238–8248. doi:10.3748/wjg.v21.i27.8238. PMC 4507093. PMID 26217075.
- Attar N, Kurdistani SK (March 2017). “Exploitation of EP300 and CREBBP Lysine Acetyltransferases by Cancer”. Cold Spring Harbor Perspectives in Medicine. 7 (3): a026534. doi:10.1101/cshperspect.a026534. PMC 5334244. PMID 27881443.
- Akinsiku OE, Soremekun OS, Soliman ME (February 2021). “Update and Potential Opportunities in CBP [Cyclic Adenosine Monophosphate (cAMP) Response Element-Binding Protein (CREB)-Binding Protein] Research Using Computational Techniques”. The Protein Journal. 40 (1): 19–27. doi:10.1007/s10930-020-09951-8. PMC7868315. PMID33394237.
- Chan HM, La Thangue NB (July 2001). “p300/CBP proteins: HATs for transcriptional bridges and scaffolds”. Journal of Cell Science. 114 (Pt 13): 2363–2373. doi:10.1242/jcs.114.13.2363. PMID 11559745.
According to the Catalog of Somatic Mutations in Cancer (COSMIC), the most common genetic mutations in CBP are missense mutations (accounting for ~71% of all CBP mutations). The most frequent mutations occur in the KAT domain, resulting largely in either decreased or inhibited acetyltransferase activity.
- Attar N, Kurdistani SK (March 2017). “Exploitation of EP300 and CREBBP Lysine Acetyltransferases by Cancer”. Cold Spring Harbor Perspectives in Medicine. 7 (3): a026534. doi:10.1101/cshperspect.a026534. PMC 5334244. PMID 27881443
| Cancer Type | N | % of Samples Mutated |
|---|---|---|
| Follicular lymphoma | 66 | 33.3 |
| Skin squamous cell carcinoma | 77 | 28.6 |
| Marginal zone B-cell lymphoma | 15 | 13.3 |
| Diffuse large B-cell lymphoma | 242 | 12.0 |
| Salivary gland carcinoma | 63 | 9.5 |
| Bladder carcinoma | 438 | 8.9 |
| Endometrial carcinoma | 337 | 8.0 |
| Lung small-cell carcinoma | 52 | 7.7 |
| ER+ Breast carcinoma | 80 | 7.5 |
Hematological malignancies
Embryonic mice heterozygous for CBP (Cbp+/-) exhibited “extramedullary hematopoiesis, decreased bone marrow cellularity [a lower ratio of bone marrow to fat], and hematopoietic differentiation abnormalities.” By age 1, these mice had an increased incidence of leukemia or hematologic neoplasia. Interestingly, tumor sequencing showed loss of heterozygosity for the wild type allele. One explanation proposed for these experimental results is that CBP plays a role in hematopoietic stem cell self-renewal.
- Dutta R, Tiu B, Sakamoto KM (September 2016). “CBP/p300 acetyltransferase activity in hematologic malignancies”. Molecular Genetics and Metabolism. 119 (1–2): 37–43. doi:10.1016/j.ymgme.2016.06.013. PMID 27380996.
- Blobel GA (2000-02-01). “CREB-binding protein and p300: molecular integrators of hematopoietic transcription”. Blood. 95 (3): 745–755. doi:10.1182/blood.V95.3.745.003k05_745_755. ISSN 1528-0020. PMID 10648382.
In cases of patients diagnosed with Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome, CBP has been shown to gain function. This occurs via chromosomal translocations between CBP and other acetyltransferase called the monocytes leukemia zinc finger (MOZ), and or between MORF (MOZ-related factor) and MLL (mixed-lineage leukemia) genes. Both instances result in fused proteins in which C-terminus of CBP is lost, and the acetyltransferase domains from both proteins remain, resulting in up-regulated KAT activity and onset of disease.
- Attar N, Kurdistani SK (March 2017). “Exploitation of EP300 and CREBBP Lysine Acetyltransferases by Cancer”. Cold Spring Harbor Perspectives in Medicine. 7 (3): a026534. doi:10.1101/cshperspect.a026534. PMC 5334244. PMID 27881443
For patients with a relapsing case of Acute Lymphoblastic Leukemia (ALL), it was reported approximately 18% of them had CBP KAT domain mutations.
- Dutta R, Tiu B, Sakamoto KM (September 2016). “CBP/p300 acetyltransferase activity in hematologic malignancies”. Molecular Genetics and Metabolism. 119 (1–2): 37–43. doi:10.1016/j.ymgme.2016.06.013. PMID 27380996.
Solid tumors
CBP mutations, though relatively infrequent, have been identified in lung cancer. Further analysis has also revealed that during the early stages of respiratory epithelium tumorigenesis, there is increased expression in CBP as well as AP-1 and cyclin D1, factors known to be associated with CBP transcriptional activity. This over-expression may lead to downstream signaling events that favor lung tumor development.
- Karamouzis MV, Konstantinopoulos PA, Papavassiliou AG (April 2007). “Roles of CREB-binding protein (CBP)/p300 in respiratory epithelium tumorigenesis”. Cell Research. 17 (4): 324–332. doi:10.1038/cr.2007.10. PMID 17372613. S2CID 36084602.
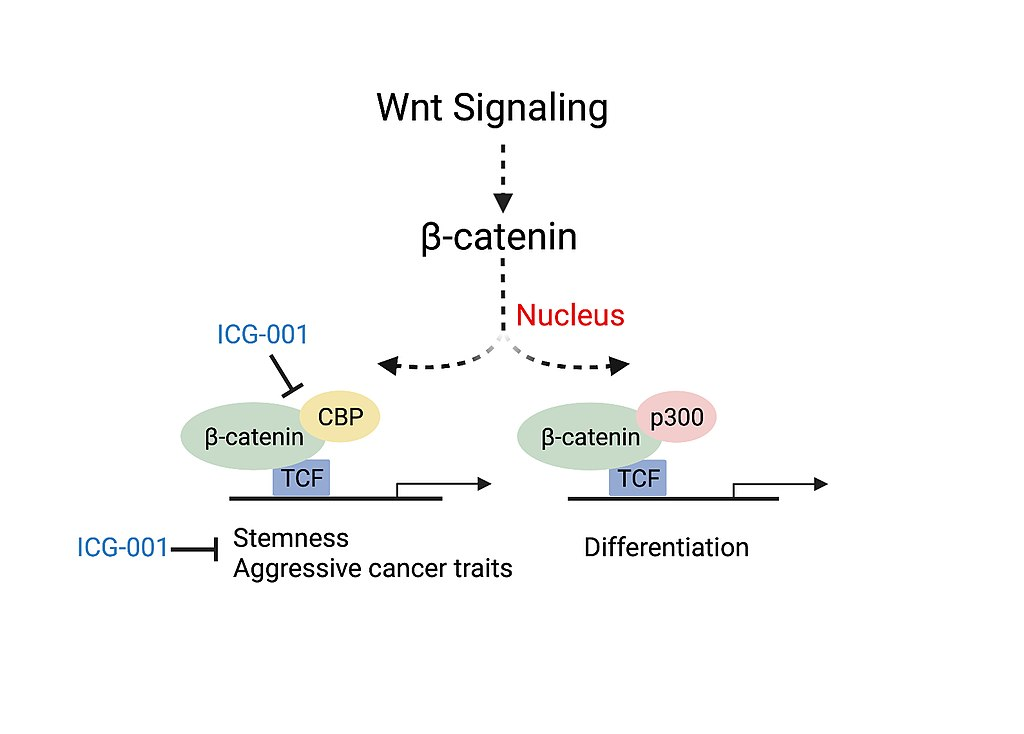
Colorectal cancer and head and neck cancer (HNSCC) disease severity have been linked to the association of CBP with β-catenin, a critical factor involved in the canonical Wnt signaling pathway. Association of CBP with β-catenin leads to the transcription of genes responsible for more aggressive cancer traits including the presence of cancer stem cell populations, decreased immune cell infiltration and likelihood of metastasis. Experiments studying the use of a small molecule inhibitor of β-catenin/CBP association (ICG-001), that does not block p300/β-catenin association, saw decreased carcinogenesis and increased cellular differentiation and apoptosis.
- Bordonaro M, Lazarova DL (July 2015). “CREB-binding protein, p300, butyrate, and Wnt signaling in colorectal cancer”. World Journal of Gastroenterology. 21 (27): 8238–8248. doi:10.3748/wjg.v21.i27.8238. PMC 4507093. PMID 26217075.
- Kartha VK, Alamoud KA, Sadykov K, Nguyen BC, Laroche F, Feng H, et al. (July 2018). “Functional and genomic analyses reveal therapeutic potential of targeting β-catenin/CBP activity in head and neck cancer”. Genome Medicine. 10 (1): 54. doi:10.1186/s13073-018-0569-7. PMC 6053793. PMID 30029671.
Increased nuclear hormone signaling, mediated by the androgen (AR) and estrogen (ER) receptors, are responsible for a number of prostate and breast cancer cases, respectively. CBP is known to interact with the AR and ER in both coactivator and acetyltransferase contexts. Inhibition of CBP KAT activity has been shown to decrease AR and ER signaling by down regulating receptor expression; this in turn suppresses tumorigenesis of both malignancies.
- Bordonaro M, Lazarova DL (July 2015). “CREB-binding protein, p300, butyrate, and Wnt signaling in colorectal cancer”. World Journal of Gastroenterology. 21 (27): 8238–8248. doi:10.3748/wjg.v21.i27.8238. PMC 4507093. PMID 26217075.

Leave a Reply