Riboflavin and its breakdown products interact with DNA, making this system attractive in the photodisinfection of blood and blood products
The application of photosensitisers to tropical pathogens in the blood supply
Mark Wainwright PhD, Mauricio S. Baptista, in Photodiagnosis and Photodynamic Therapy, 2011
Riboflavin
As vitamin B2, riboflavin (Fig. 5) is an essential nutrient in humans. The Mirasol system (Navigant Biotechnologies Inc., CO, USA) utilises riboflavin as a photosensitiser in conjunction with long-wave ultraviolet light [37].
Plainly given its essential nature, there are fewer potential toxicity problems with riboflavin than with completely synthetic xenobiotics [38].
A xenobiotic is a chemical substance found within an organism that is not naturally produced or expected to be present within the organism. It can also cover substances that are present in much higher concentrations than are usual. Natural compounds can also become xenobiotics if they are taken up by another organism, such as the uptake of natural human hormones by fish found downstream of sewage treatment plant outfalls, or the chemical defenses produced by some organisms as protection against predators.[Mansuy D (2013). “Metabolism of xenobiotics: beneficial and adverse effects”. Biol Aujourdhui. 207 (1): 33–37. doi:10.1051/jbio/2013003. PMID 23694723.] The term xenobiotics, however, is very often used in the context of pollutants such as dioxins and polychlorinated biphenyls and their effect on the biota, because xenobiotics are understood as substances foreign to an entire biological system, i.e. artificial substances, which did not exist in nature before their synthesis by humans. The term xenobiotic is derived from the Greek words ξένος (xenos) = foreigner, stranger and βίος (bios) = life, plus the Greek suffix for adjectives -τικός, -ή, -όν (-tikos, -ē, -on). Xenobiotics may be grouped as carcinogens, drugs, environmental pollutants, food additives, hydrocarbons, and pesticides. The body removes xenobiotics by xenobiotic metabolism. This consists of the deactivation and the excretion of xenobiotics and happens mostly in the liver. Excretion routes are urine, feces, breath, and sweat. Hepatic enzymes are responsible for the metabolism of xenobiotics by first activating them (oxidation, reduction, hydrolysis, and/or hydration of the xenobiotic), and then conjugating the active secondary metabolite with glucuronic acid, sulfuric acid, or glutathione, followed by excretion in bile or urine. An example of a group of enzymes involved in xenobiotic metabolism is hepatic microsomal cytochrome P450. These enzymes that metabolize xenobiotics are very important for the pharmaceutical industry because they are responsible for the breakdown of medications. A species with this unique cytochrome P450 system is Drosophila mettleri, which uses xenobiotic resistance to exploit a wider nesting range including both soil moistened with necrotic exudates and necrotic plots themselves.Although the body is able to remove xenobiotics by reducing it to a less toxic form through xenobiotic metabolism then excreting it, it is also possible for it to be converted into a more toxic form in some cases. This process is referred to as bioactivation and can result in structural and functional changes to the microbiota.[Park, B.K.; Laverty, H.; Srivastava, A.; Antoine, D.J.; Naisbitt, D.; Williams, D.P. (2011). “Drug bioactivation and protein adduct formation in the pathogenesis of drug-induced toxicity”. Chemico-Biological Interactions. 192 (1–2): 30–36. doi:10.1016/j.cbi.2010.09.011. PMID 20846520.] Exposure to xenobiotics can disrupt the microbiome community structure, either by increasing or decreasing the size of certain bacterial populations depending on the substance. Functional changes that result vary depending on the substance and can include increased expression in genes involved in stress response and antibiotic resistance, changes in the levels of metabolites produced, etc.[Lu, Kun; Mahbub, Ridwan; Fox, James G. (31 August 2015). “Xenobiotics: Interaction with the Intestinal Microflora”. ILAR Journal. 56 (2): 218–227. doi:10.1093/ilar/ilv018. ISSN 1084-2020. PMC 4654756. PMID 26323631.] See also Drug metabolism – Xenobiotic metabolism is redirected to the special case: Drug metabolism.

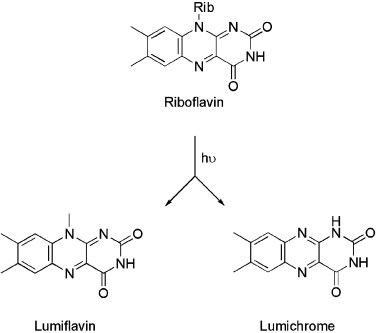
The photochemistry associated with riboflavin, breaking down into lumiflavin and lumichrome [39] is well understood, and the photoproducts, each of which is also a photosensitiser, are non-toxic to humans [40]. Riboflavin and its breakdown products interact with DNA, making this system attractive in the photodisinfection of blood and blood products [41].
Photosensitizers are light absorbers that alters the course of a photochemical reaction. They usually are catalysts.[ “Photosensitization”. IUPAC Gold Book. International Union of Pure and Applied Chemistry.] They can function by many mechanisms, sometimes they donate an electron to the substrate, sometimes they abstract a hydrogen atom from the substrate. At the end of this process, the photosensitizer returns to its ground state, where it remains chemically intact, poised to absorb more light.[Gómez Alvarez E, Wortham H, Strekowski R, Zetzsch C, Gligorovski S (February 2012). “Atmospheric photosensitized heterogeneous and multiphase reactions: from outdoors to indoors”. Environmental Science & Technology. 46 (4): 1955–63. Bibcode:2012EnST…46.1955G. doi:10.1021/es2019675. PMID 22148293.][Zhang Y, Lee TS, Petersen JL, Milsmann C (May 2018). “A Zirconium Photosensitizer with a Long-Lived Excited State: Mechanistic Insight into Photoinduced Single-Electron Transfer”. Journal of the American Chemical Society. 140 (18): 5934–5947. doi:10.1021/jacs.8b00742. PMID 29671586.][“Photosensitization”. IUPAC Compendium of Chemical Terminology. 2009. doi:10.1351/goldbook.P04652. ISBN 978-0-9678550-9-7.] One branch of chemistry which frequently utilizes photosensitizers is polymer chemistry, using photosensitizers in reactions such as photopolymerization, photocrosslinking, and photodegradation.[Alger M (1996). Polymer science dictionary (2nd ed.). London: Chapman & Hall. ISBN 978-0412608704.] Photosensitizers are also used to generate prolonged excited electronic states in organic molecules with uses in photocatalysis, photon upconversion and photodynamic therapy. Generally, photosensitizers absorb electromagnetic radiation consisting of infrared radiation, visible light radiation, and ultraviolet radiation and transfer absorbed energy into neighboring molecules. This absorption of light is made possible by photosensitizers’ large de-localized π-systems, which lowers the energy of HOMO and LUMO orbitals to promote photoexcitation. While many photosensitizers are organic or organometallic compounds, there are also examples of using semiconductor quantum dots as photosensitizers.[Liu Y, Ma Y, Zhao Y, Sun X, Gándara F, Furukawa H, et al. (January 2016). “Weaving of organic threads into a crystalline covalent organic framework”. Science. 351 (6271): 365–9. Bibcode:2016Sci…351..365L. doi:10.1126/science.aad4011. PMID 26798010.] Photosensitizers that are readily incorporated into the external tissues can increase the rate at which reactive oxygen species are generated upon exposure to UV light (such as UV-containing sunlight). Some photosensitizing agents, such as St. John’s Wort, appear to increase the incidence of inflammatory skin conditions in animals and have been observed to slightly reduce the minimum tanning dose in humans.[ Kumper H. [Hypericum poisoning in sheep]. Tierarztl Prax 1989;17:257-261.][Brockmoller J, et al. Hypericin and pseudohypericin: Pharmacokinetics and effects on photosensitivity in humans. Pharmacopsychiatry 1997;30(Suppl 2): 94-101.] Some examples of photosensitizing medications (both investigatory and approved for human use) are: St. John’s Wort[Brockmoller J, et al. Hypericin and pseudohypericin: Pharmacokinetics and effects on photosensitivity in humans. Pharmacopsychiatry 1997;30(Suppl 2): 94-101.] 9-me-bc[Vignoni, Mariana; Rasse-Suriani, Federico A. O.; Butzbach, Kathrin; Erra-Balsells, Rosa; Epe, Bernd; Cabrerizo, Franco M. (2013-07-24). “Mechanisms of DNA damage by photoexcited 9-methyl-β-carbolines”. Organic & Biomolecular Chemistry. 11 (32): 5300–5309.] Doxepin[“Medications and other Agents that Increase Sensitivity to Light”. Wisconsin Department of Health Services. 2013-07-11. Retrieved 2022-11-01.] Amoxapine[“Medications and other Agents that Increase Sensitivity to Light”. Wisconsin Department of Health Services. 2013-07-11. Retrieved 2022-11-01.] Ethinyl estradiol[“Medications and other Agents that Increase Sensitivity to Light”. Wisconsin Department of Health Services. 2013-07-11. Retrieved 2022-11-01.]
The reported photoantimicrobial activity of riboflavin is significant, covering a range of Gram-positive and Gram-negative bacteria, and viruses important in blood products, such as HIV-1, cytomegalovirus and West Nile virus [37]. In terms of more typical tropical pathogens, both Leishmania donovani infantum, T. cruzi and Plasmodia spp. were effectively killed in both plasma and platelet fractions using riboflavin and ultraviolet light [42–44]. Riboflavin use in red cells is currently under development [7].
References (60)
[1] Mascarello M, Gobbi F, Angheben A, et al. Imported malariain immigrants to Italy: a changing pattern observed in northeastern Italy. J Travel Med 2009;16:317—21.
[2] Mu˜noz J, Gómez i Prat J, Gállego M, et al. Clinical profile of Trypanosoma cruzi infection in a non-endemic setting: immi-gration and Chagas disease in Barcelona (Spain). Acta Trop2009;111:51—5.
[3] Grellier P, Santus R, Mouray E, et al. Photosensitized inactivation of Plasmodium falciparum-and Babesia divergens-infected erythrocytes in whole blood by lipophilic pheophor-bide derivatives. Vox Sang 1997;72:211—20
[4] Garraud O. Mechanisms of transfusion-linked parasite infection. Transfus Clin Biol 2006;13:290—7.[
5] Kurtis JD, Strobl FJ. Other transfusion-transmitted infections.In: Hillyer CD, Hillyer KI, Strobl FJ, Jefferies LC, SilbersteinLE, editors. Handbook of transfusion medicine. New York: Aca-demic Press; 2001. p. 301—6.
[8] Zavizion B, Pereira M, de Melo JM, et al. Inactivation of pro-tozoan parasites in red blood cells using INACTINE PEN110chemistry. Transfusion (Paris) 2004;44:731—8.
[10] Wainwright M. Photodynamic antimicrobial chemotherapy(PACT). J Antimicrob Chemother 1998;42:13—28.
[11] Raab OZ. Uber die wirking fluoreszierender stoffe auf infu-sorien. Z Biol 1900;39:524—46.
[13] T’ung T. Photodynamic action of methylene blue on bacteria.Proc Soc Exp Biol Med 1935;33:328—30.
[14] T’ung T. In vitro photodynamic action of methylene blue on Trypanosoma brucei. Proc Soc Exp Biol Med 1938;38:29—31.
[16] Engelmann FM, Mayer I, Gabrielli D, Toma HE, Kowaltowski AJ.Interactions of cationic meso-porphyrins with biomembranes.J Bioenerg Biomembr 2007;39:175—85.
[23] Van Steveninck J, Trannoy LL, Besselink GA, et al. Selective protection of RBCs against photodynamic damage by the band3 ligand dipyramidole. Transfusion (Paris) 2000;40:1330—6.
[24] Besselink GA, van Engelenburg FA, Ebbing IG, Hilarius PM,de Korte D, Verhoeven AJ. Additive effects of dipyramidoleand Trolox in protecting human red cells during photodynamictreatment. Vox Sang 2003;85:25—30.
[25] Schultz EW, Krueger AP. Inactivation of Staphylococcus bac-teriophage by methylene blue. Proc Soc Exp Biol Med1928;26:100—1.
[29] FindlayGM.Immunization against yellow fever.TransRSocTropMed Hyg 1934;27:437—69.
[34] Bountogo M, Zoungrana A, Coulibaly B, et al. Efficacy of methylene blue monotherapy in semi-immune adults withuncomplicated falciparum malaria: a controlled trial in BurkinaFaso. Trop Med Int Health 2010;15:713—7.
[35] Rampersad J, Cesar E, Campbell MD, Samlal M, Ammons D. Afield evaluation of PCR for the routine detection of Babesiaequi in horses. Vet Parasitol 2003;114:81—7.
[36] Wainwright M. Ring-fused derivatives of 3,7-diaminophenothiazinium and phenoselenazinium salts foruse as antimicrobial agents. GB Pat 2 2009;453:564.
[37] Goodrich RP, Edrich RA, Li J, Seghatchian J. The Mirasol TMPRT system for pathogen reduction of platelets and plasma: an overview of current status and future trends. Transfus ApherSci 2006;35:5—17.
42] Cardo LJ, Rentas FJ, Ketchum L, et al. Pathogen inactiva-tion of Leishmania donovani infantum in plasma and plateletconcentrates using riboflavin and ultraviolet light. Vox Sang2006;90:85—91.
[44] Reddy H, Goodrich RP. Inactivation of West Nile virus andmalariausingphotosensitizers.VoxSang2003.WO2003063902.
[48] Gottlieb P, Shen LG, Chimezie E, et al. Inactivation of Try- panosoma cruzi trypomastigote forms in blood componentsby photodynamic treatment with phthalocyanines. PhotochemPhotobiol 1995;62:869—74.
[50] Trannoy LL, Terpstra FG, de Korte D, et al. Differentialsensitivities of pathogens in red cell concentrates to Tri-P(4)-photoinactivation. Vox Sang 2006;91:111—8.
[51] Zupan K, Egyeki M, Toth K, et al. Comparison of the efficiencyand the specificity of DNA-bound and free cationic porphyrinin photodynamic virus inactivation. J Photochem Photobiol B2008;90:105—12.
[54] Castro E, Gironés N, Bueno JL, Carrión J, Lin L, Fresno M. The efficacy of photochemical treatment with amotosalenHCl and ultraviolet A (INTERCEPT) for inactivation of Try- panosoma cruzi in pooled buffy-coat platelets. Transfusion(Paris) 2007;47:434—41.
[55] McCullough J, Vesole DH, Benjamin RJ, et al. Therapeuticefficacy and safety of platelets treated with a photochemi-cal process for pathogen inactivation: the SPRINT trial. Blood2004;104:1534—41.
[58] Prowse C. Properties of pathogen-inactivated plasma components. Transfus Med Rev 2009;23:124—33.


Leave a Reply