Fibroin is an insoluble protein present in silk produced by numerous insects
Fibroin is an insoluble protein present in silk produced by numerous insects, such as the larvae of Bombyx mori, and other moth genera such as Antheraea, Cricula, Samia and Gonometa. Silk in its raw state consists of two main proteins, sericin and fibroin, with a glue-like layer of sericin coating two singular filaments of fibroin called brins.
BRIN AND BAVE (BRIN) One of the radiating sticks of a fan. The outermost are larger and longer, and are called panaches[1874, Edward H. Knight, American Mechanical Dictionary]. A single silkworm thread extruded from the gland, before it has formed a bave. Unknown origin; possibly of Gaulish origin (compare Catalan bri, Spanish brenca (“fiber”), brinza (“blade of grass, filament”)), from Proto-Celtic *brinikā, from *brinos (“filament, fiber”) (compare Breton broenenn, Welsh brwynen), from Proto-Indo-European *bʰrugh-no- (“twig”), perhaps related to the root of English brush.[en.wiktionary.org/wiki/brin] (BAVE) silkworm thread extruded as brins from the two glands and stuck together with sericin proteins. From Old French beve (altered based on baver), from Vulgar Latin root *baba, of imitative origin.[https://en.wiktionary.org/wiki/bave] These words link to brimstone and beaver at my favorite etymology website and that makes me giggle just a little (Vulgar English)..so connections to stones, sulfur, amber and saliva.
- Hakimi O, Knight DP, Vollrath F, Vadgama P (April 2007). “Spider and mulberry silkworm silks as compatible biomaterials”. Composites Part B: Engineering. 38 (3): 324–37. doi:10.1016/j.compositesb.2006.06.012.
- Dyakonov T, Yang CH, Bush D, Gosangari S, Majuru S, Fatmi A (2012). “Design and characterization of a silk-fibroin-based drug delivery platform using naproxen as a model drug”. Journal of Drug Delivery. 2012: 490514. doi:10.1155/2012/490514. PMC 3312329. PMID 22506122.
- “Brin definition and meaning | Collins English Dictionary”. www.collinsdictionary.com.
Silk fibroin is considered a β-keratin related to proteins that form hair, skin, nails and connective tissues.
The silk worm produces fibroin with three chains, the light, heavy, and the glycoprotein P25. The heavy and light chains are linked by a disulphide bond, and P25 associates with disulphide-linked heavy and light chains by noncovalent interactions. P25 plays an important role in maintaining integrity of the complex.
- Inoue S, Tanaka K, Arisaka F, Kimura S, Ohtomo K, Mizuno S (December 2000). “Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio”. The Journal of Biological Chemistry. 275 (51): 40517–28. doi:10.1074/jbc.M006897200. PMID 10986287.
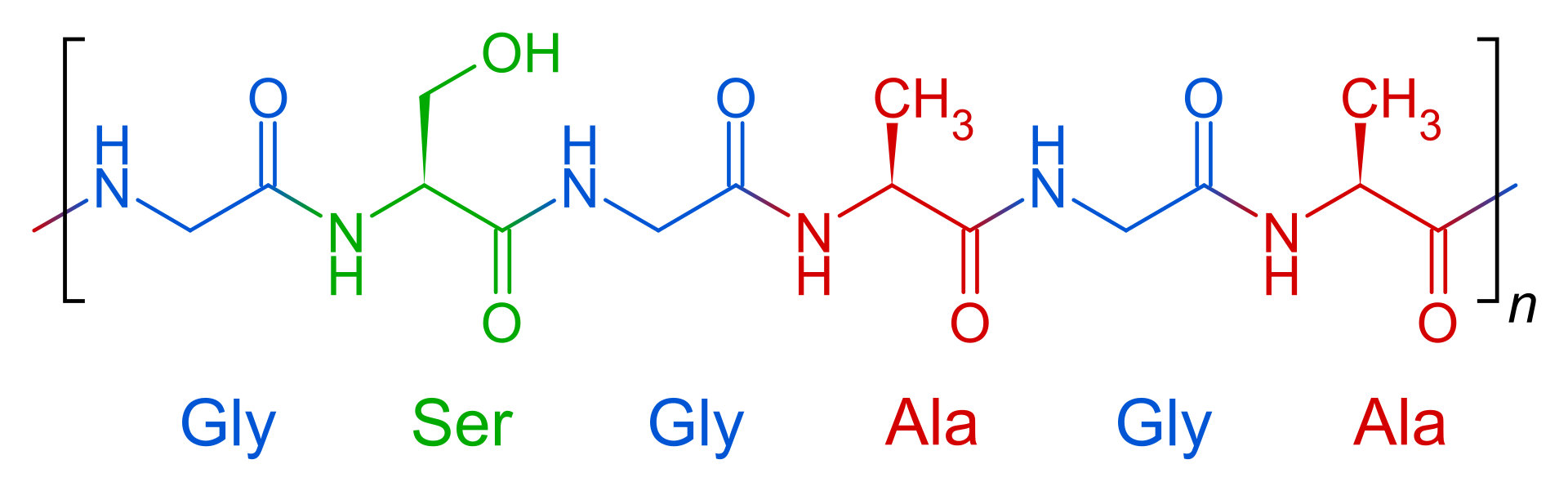
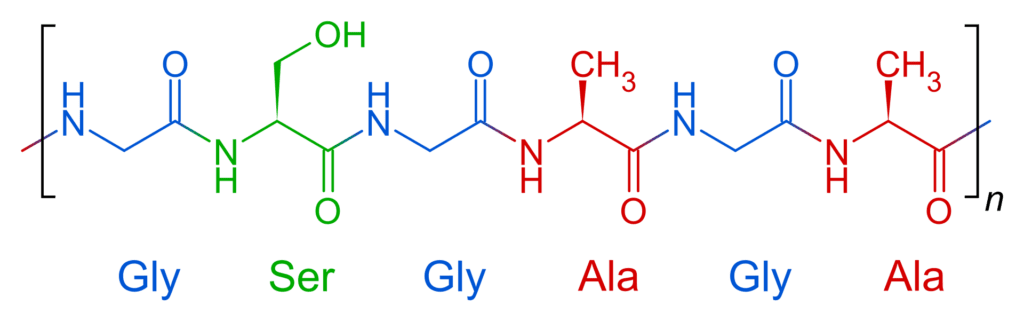
The heavy fibroin protein consists of layers of antiparallel beta sheets. Its primary structure mainly consists of the recurrent amino acid sequence (Gly–Ser-Gly-Ala-Gly-Ala)n. The high glycine (and, to a lesser extent, alanine) content allows for tight packing of the sheets, which contributes to silk’s rigid structure and tensile strength. A combination of stiffness and toughness make it a material with applications in several areas, including biomedicine and textile manufacture.
Fibroin is known to arrange itself in three structures, called silk I, II, and III. Silk I is the natural form of fibroin, as emitted from the Bombyx mori silk glands. Silk II refers to the arrangement of fibroin molecules in spun silk, which has greater strength and is often used in various commercial applications. Silk III is a newly discovered structure of fibroin.
- Valluzzi R, Gido SP, Muller W, Kaplan DL (1999). “Orientation of silk III at the air-water interface”. International Journal of Biological Macromolecules. 24 (2–3): 237–42. doi:10.1016/S0141-8130(99)00002-1
Silk III is formed principally in solutions of fibroin at an interface (i.e. air-water interface, water-oil interface, etc.).
Degradation
Many species of Amycolatopsis and Saccharotrix bacteria are able to degrade both silk fibroin and polylactic acid.
- Tokiwa Y, Calabia BP, Ugwu CU, Aiba S (August 2009). “Biodegradability of plastics”. International Journal of Molecular Sciences. 10 (9): 3722–42. doi:10.3390/ijms10093722. PMC 2769161. PMID 19865515.
Polylactic acid, also known as poly(lactic acid) or polylactide (PLA), is a thermoplastic polyester with backbone formula (C3H4O2)n or [–C(CH3)HC(=O)O–]n, formally obtained by condensation of lactic acid C(CH3)(OH)HCOOH with loss of water (hence its name). It can also be prepared by ring-opening polymerization of lactide [–C(CH3)HC(=O)O–]2, the cyclic dimer of the basic repeating unit. PLA has become a popular material due to it being economically produced from renewable resources. In 2021, PLA had the highest consumption volume of any bioplastic of the world,[Ceresana. “Bioplastics – Study: Market, Analysis, Trends – Ceresana”. www.ceresana.com. Archived from the original on 4 November 2017. Retrieved 22 November 2022.] although it is still not a commodity polymer. Its widespread application has been hindered by numerous physical and processing shortcomings.[Nagarajan V, Mohanty AK, Misra M (2016). “Perspective on Polylactic Acid (PLA) based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance”. ACS Sustainable Chemistry & Engineering. 4 (6): 2899–2916. doi:10.1021/acssuschemeng.6b00321.] PLA is the most widely used plastic filament material in 3D printing. Its low melting point, high strength, low thermal expansion, good layer adhesion, and high heat resistance when annealed make it an ideal material for this purpose. Without annealing, however, PLA has the lowest heat resistance of the common 3D printing plastics. Although the name “polylactic acid” is widely used, it does not comply with IUPAC standard nomenclature, which is “poly(lactic acid)”.[Vert M, Chen J, Hellwich KH, Hodge P, Nakano T, Scholz C, Slomkowski S, Vohlidal J. “Nomenclature and Terminology for Linear Lactic Acid-Based Polymers (IUPAC Recommendations 2019)”. IUPAC Standards Online. doi:10.1515/iupac.92.0001.] The name “polylactic acid” is potentially ambiguous or confusing, because PLA is not a polyacid (polyelectrolyte), but rather a polyester.[Martin O, Avérous L (2001). “Poly(lactic acid): plasticization and properties of biodegradable multiphase systems”. Polymer. 42 (14): 6209–6219. doi:10.1016/S0032-3861(01)00086-6.]
CHEMICAL PROPERTIES – SYNTHESIS – The monomer is typically made from fermented plant starch such as from corn, cassava, sugarcane or sugar beet pulp. Several industrial routes afford usable (i.e. high molecular weight) PLA. Two main monomers are used: lactic acid, and the cyclic di-ester, lactide. The most common route to PLA is the ring-opening polymerization of lactide with various metal catalysts (typically tin octoate) in solution or as a suspension. The metal-catalyzed reaction tends to cause racemization of the PLA, reducing its stereoregularity compared to the starting material (usually corn starch).[Södergård A, Stolt M (2010). “3. Industrial Production of High Molecular Weight Poly(Lactic Acid)”. In Auras R, Lim LT, Selke SE, Tsuji H (eds.). Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications. pp. 27–41. doi:10.1002/9780470649848.ch3. ISBN 978-0-470-64984-8.] The direct condensation of lactic acid monomers can also be used to produce PLA. This process needs to be carried out at less than 200 °C; above that temperature, the entropically favored lactide monomer is generated. This reaction generates one equivalent of water for every condensation (esterification) step. The condensation reaction is reversible and subject to equilibrium, so removal of water is required to generate high molecular weight species. Water removal by application of a vacuum or by azeotropic distillation is required to drive the reaction toward polycondensation. Molecular weights of 130 kDa can be obtained this way. Even higher molecular weights can be attained by carefully crystallizing the crude polymer from the melt. Carboxylic acid and alcohol end groups are thus concentrated in the amorphous region of the solid polymer, and so they can react. Molecular weights of 128–152 kDa are obtainable thus.[Södergård A, Stolt M (2010). “3. Industrial Production of High Molecular Weight Poly(Lactic Acid)”. In Auras R, Lim LT, Selke SE, Tsuji H (eds.). Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications. pp. 27–41. doi:10.1002/9780470649848.ch3. ISBN 978-0-470-64984-8.] Another method devised is by contacting lactic acid with a zeolite. This condensation reaction is a one-step process, and runs about 100 °C lower in temperature.[Drury J (15 February 2016). “Cheaper, greener, route to bioplastic”. reuters.com. Archived from the original on 1 December 2017. Retrieved 9 May 2018.][Dusselier M, Van Wouwe P, Dewaele A, Jacobs PA, Sels BF (July 2015). “GREEN CHEMISTRY. Shape-selective zeolite catalysis for bioplastics production”. Science. 349 (6243): 78–80. Bibcode:2015Sci…349…78D. doi:10.1126/science.aaa7169. PMID 26138977. S2CID 206635718.]
STEREOISOMERS – Due to the chiral nature of lactic acid, several distinct forms of polylactide exist: poly-L-lactide (PLLA) is the product resulting from polymerization of L,L-lactide (also known as L-lactide). Progress in biotechnology has resulted in the development of commercial production of the D enantiomer form.[“Bioengineers succeed in producing plastic without the use of fossil fuels”. Physorg.com. Archived from the original on 6 June 2011. Retrieved 11 April 2011.] Polymerization of a racemic mixture of L– and D-lactides usually leads to the synthesis of poly-DL-lactide (PDLLA), which is amorphous. Use of stereospecific catalysts can lead to heterotactic PLA which has been found to show crystallinity. The degree of crystallinity, and hence many important properties, is largely controlled by the ratio of D to L enantiomers used, and to a lesser extent on the type of catalyst used. Apart from lactic acid and lactide, lactic acid O-carboxyanhydride (“lac-OCA”), a five-membered cyclic compound has been used academically as well. This compound is more reactive than lactide, because its polymerization is driven by the loss of one equivalent of carbon dioxide per equivalent of lactic acid. Water is not a co-product.[Kricheldorf HR, Jonté JM (1983). “New polymer syntheses”. Polymer Bulletin. 9 (6–7). doi:10.1007/BF00262719. S2CID 95429767.] The direct biosynthesis of PLA, in a manner similar to production of poly(hydroxyalkanoate)s, has been reported.[Jung YK, Kim TY, Park SJ, Lee SY (January 2010). “Metabolic engineering of Escherichia coli for the production of polylactic acid and its copolymers”. Biotechnology and Bioengineering. 105 (1): 161–171. doi:10.1002/bit.22548. PMID 19937727. S2CID 205499487.]
PHYSICAL PROPERTIES – PLA polymers range from amorphous glassy polymer to semi-crystalline and highly crystalline polymer with a glass transition 60–65 °C, a melting temperature 130-180 °C, and a Young’s modulus 2.7–16 GPa.[Lunt J (3 January 1998). “Large-scale production, properties and commercial applications of polylactic acid polymers”. Polymer Degradation and Stability. 59 (1–3): 145–152. doi:10.1016/S0141-3910(97)00148-1. ISSN 0141-3910.][Södergård A, Stolt M (February 2002). “Properties of lactic acid based polymers and their correlation with composition”. Progress in Polymer Science. 27 (6): 1123–1163. doi:10.1016/S0079-6700(02)00012-6.][Middleton JC, Tipton AJ (December 2000). “Synthetic biodegradable polymers as orthopedic devices”. Biomaterials. 21 (23): 2335–2346. doi:10.1016/S0142-9612(00)00101-0. PMID 11055281.] Heat-resistant PLA can withstand temperatures of 110 °C.[Fiore GL, Jing F, Young Jr VG, Cramer CJ, Hillmyer MA (2010). “High Tg Aliphatic Polyesters by the Polymerization of Spirolactide Derivatives”. Polymer Chemistry. 1 (6): 870–877. doi:10.1039/C0PY00029A.] The basic mechanical properties of PLA are between those of polystyrene and PET.[Lunt J (3 January 1998). “Large-scale production, properties and commercial applications of polylactic acid polymers”. Polymer Degradation and Stability. 59 (1–3): 145–152. doi:10.1016/S0141-3910(97)00148-1. ISSN 0141-3910.] The melting temperature of PLLA can be increased by 40–50 °C and its heat deflection temperature can be increased from approximately 60 °C to up to 190 °C by physically blending the polymer with PDLA (poly-D-lactide). PDLA and PLLA form a highly regular stereocomplex with increased crystallinity. The temperature stability is maximised when a 1:1 blend is used, but even at lower concentrations of 3–10% of PDLA, there is still a substantial improvement. In the latter case, PDLA acts as a nucleating agent, thereby increasing the crystallization rate.[Park HS, Hong CK (June 2021). “Relationship between the Stereocomplex Crystallization Behavior and Mechanical Properties of PLLA/PDLA Blends”. Polymers. 13 (11): 1851. doi:10.3390/polym13111851. PMC 8199684. PMID 34199577.] Biodegradation of PDLA is slower than for PLA due to the higher crystallinity of PDLA[citation needed]. The flexural modulus of PLA is higher than polystyrene and PLA has good heat sealability. Several technologies such as annealing,[Nugroho P, Mitomo H, Yoshii F, Kume T (1 May 2001). “Degradation of poly(l-lactic acid) by γ-irradiation”. Polymer Degradation and Stability. 72 (2): 337–343. doi:10.1016/S0141-3910(01)00030-1. ISSN 0141-3910.][Urayama H, Kanamori T, Fukushima K, Kimura Y (1 September 2003). “Controlled crystal nucleation in the melt-crystallization of poly(l-lactide) and poly(l-lactide)/poly(d-lactide) stereocomplex”. Polymer. 44 (19): 5635–5641. doi:10.1016/S0032-3861(03)00583-4. ISSN 0032-3861.][Tsuji H (1 January 1995). “Properties and morphologies of poly(l-lactide): 1. Annealing condition effects on properties and morphologies of poly(l-lactide)”. Polymer. 36 (14): 2709–2716. doi:10.1016/0032-3861(95)93647-5. ISSN 0032-3861.] adding nucleating agents, forming composites with fibers or nano-particles,[Urayama H, Ma C, Kimura Y (July 2003). “Mechanical and Thermal Properties of Poly(L-lactide) Incorporating Various Inorganic Fillers with Particle and Whisker Shapes”. Macromolecular Materials and Engineering. 288 (7): 562–568. doi:10.1002/mame.200350004. ISSN 1438-7492.][Trimaille T, Pichot C, Elaissari A, Fessi H, Briançon S, Delair T (1 November 2003). “Poly(d,l-lactic acid) nanoparticle preparation and colloidal characterization”. Colloid and Polymer Science. 281 (12): 1184–1190. doi:10.1007/s00396-003-0894-1. ISSN 0303-402X. S2CID 98078359.][Hu X, Xu HS, Li ZM (4 May 2007). “Morphology and Properties of Poly(L-Lactide) (PLLA) Filled with Hollow Glass Beads”. Macromolecular Materials and Engineering. 292 (5): 646–654. doi:10.1002/mame.200600504. ISSN 1438-7492.] chain extending[Li BH, Yang MC (2006). “Improvement of thermal and mechanical properties of poly(L-lactic acid) with 4,4-methylene diphenyl diisocyanate”. Polymers for Advanced Technologies. 17 (6): 439–443. doi:10.1002/pat.731. ISSN 1042-7147. S2CID 98536537.][Di Y, Iannace S, Di Maio E, Nicolais L (4 November 2005). “Reactively Modified Poly(lactic acid): Properties and Foam Processing”. Macromolecular Materials and Engineering. 290 (11): 1083–1090. doi:10.1002/mame.200500115. ISSN 1438-7492.] and introducing crosslink structures have been used to enhance the mechanical properties of PLA polymers. Polylactic acid can be processed like most thermoplastics into fiber (for example, using conventional melt spinning processes) and film. PLA has similar mechanical properties to PETE polymer, but has a significantly lower maximum continuous use temperature.[“Compare Materials: PLA and PETE”. Makeitfrom.com. Archived from the original on 1 May 2011. Retrieved 11 April 2011.] Racemic PLA and pure PLLA have low glass transition temperatures, making them undesirable because of low strength and melting point. A stereocomplex of PDLA and PLLA has a higher glass transition temperature, lending it more mechanical strength.[Luo F, Fortenberry A, Ren J, Qiang Z (20 August 2020). “Recent Progress in Enhancing Poly(Lactic Acid) Stereocomplex Formation for Material Property Improvement”. Frontiers in Chemistry. 8: 688. Bibcode:2020FrCh….8..688L. doi:10.3389/fchem.2020.00688. PMC 7468453. PMID 32974273.] The high surface energy of PLA results in good printability, making it widely used in 3D printing. The tensile strength for 3D printed PLA was previously determined.[Giordano RA, Wu BM, Borland SW, Cima LG, Sachs EM, Cima MJ (1997). “Mechanical properties of dense polylactic acid structures fabricated by three dimensional printing”. Journal of Biomaterials Science. Polymer Edition. 8 (1): 63–75. doi:10.1163/156856297×00588. PMID 8933291.]
SOLVENTS – PLA is soluble in a range of organic solvents.[Sato S, Gondo D, Wada T, Kanehashi S, Nagai K (2013). “Effects of Various Liquid Organic Solvents on Solvent-Induced Crystallization of AMorphous Poly(lactic acid) Film”. Journal of Applied Polymer Science. 129 (3): 1607–1617. doi:10.1002/app.38833.] Ethyl acetate is widely used because of its ease of access and low risk. It is useful in 3D printers for cleaning the extruder heads and for removing PLA supports. Other safe solvents include propylene carbonate, which is safer than ethyl acetate but is difficult to purchase commercially. Pyridine can be used, but it has a distinct fish odor and is less safe than ethyl acetate. PLA is also soluble in hot benzene, tetrahydrofuran, and dioxane.[Garlotta D (2001). “A Literature Review of Poly(Lactic Acid)”. Journal of Polymers and the Environment. 9 (2): 63–84. doi:10.1023/A:1020200822435. S2CID 8630569. Archived from the original on 26 May 2013.]
FABRICATION – PLA objects can be fabricated by 3D printing, casting, injection moulding, extrusion, machining, and solvent welding. PLA is used as a feedstock material in desktop fused filament fabrication by 3D printers, such as RepRap printers.[“PLA”. Reprap Wiki. 4 April 2011. Archived from the original on 16 July 2011. Retrieved 11 April 2011.][“PLA”. MakerBot Industries. Archived from the original on 23 April 2011. Retrieved 11 April 2011.] PLA can be solvent welded using dichloromethane.[Coysh A (12 April 2013). “Dichloromethane Vapor Treating PLA parts”. Thingiverse.com. Archived from the original on 1 December 2017. Retrieved 9 May 2018.] Acetone also softens the surface of PLA, making it sticky without dissolving it, for welding to another PLA surface.[Sanladerer T (9 December 2016). “Does Acetone also work for welding and smoothing PLA 3D printed parts?”. youtube.com. Archived from the original on 21 December 2021. Retrieved 9 January 2021.] PLA-printed solids can be encased in plaster-like moulding materials, then burned out in a furnace, so that the resulting void can be filled with molten metal. This is known as “lost PLA casting”, a type of investment casting.[“Metal Casting with Your 3D Printer”. Make: DIY Projects and Ideas for Makers. Retrieved 30 November 2018.]
- Investment casting is an industrial process based on lost-wax casting, one of the oldest known metal-forming techniques.[Investment Casting Process Description] The term “lost-wax casting” can also refer to modern investment casting processes. Investment casting has been used in various forms for the last 5,000 years. In its earliest forms, beeswax was used to form patterns necessary for the casting process. Today, more advanced waxes, refractory materials and specialist alloys are typically used for making patterns. Investment casting is valued for its ability to produce components with accuracy, repeatability, versatility and integrity in a variety of metals and high-performance alloys. The fragile wax patterns must withstand forces encountered during the mould making. Much of the wax used in investment casting can be reclaimed and reused.[Kalpakjian & Schmid 2006.] Lost-foam casting is a modern form of investment casting that eliminates certain steps in the process. Investment casting is so named because the process invests (surrounds) the pattern with refractory material to make a mould, and a molten substance is cast into the mold. Materials that can be cast include stainless steel alloys, brass, aluminium, carbon steel and glass. The cavity inside the refractory mould is an exact duplicate of the desired part. Due to the hardness of refractory materials used, investment casting can produce products with exceptional surface qualities, which can reduce the need for secondary machine processes.[Investment Castings] Water glass and silica sol investment casting are the two primary investment casting methods currently in use. The main differences are the surface roughness and cost of casting. Water glass method dewaxes into the high-temperature water, and the ceramic mould is made of water glass quartz sand. Silica sol method dewaxes into the flash fire, and silica sol zircon sand makes the ceramic mould. Silica sol method costs more but has the better surface than the water glass method.[ “Investment casting”. Retrieved 2017-10-10.] The process can be used for both small castings of a few ounces and large castings weighing several hundred pounds. It can be more expensive than die casting or sand casting, but per-unit costs decrease with large volumes. Investment casting can produce complicated shapes that would be difficult or impossible with other casting methods. It can also produce products with exceptional surface qualities and low tolerances with minimal surface finishing or machining required. HISTORY The history of lost-wax casting dates back thousands of years.[ “The long history of lost wax casting. Over five thousand years of art and craftsmanship – ITRI – Tin Markets, Technology and Sustainability”. www.itri.co.uk. Retrieved 2016-06-09.] Its earliest use was for idols, ornaments and jewellery, using natural beeswax for patterns, clay for the moulds and manually operated bellows for stoking furnaces. Examples have been found across the world, such as in the Harappan Civilisation (2500–2000 BC) idols, Egypt‘s tombs of Tutankhamun (1333–1324 BC), Mesopotamia, Aztec and Mayan Mexico, and the Benin civilization in Africa where the process produced detailed artwork of copper, bronze and gold. By far, one of the earliest identified uses of the investment casting process was seen in objects found in the ‘Cave of Treasure’, discovered in Southern Israel. These items were identified as being made around 3700 BC using Carbon-14 dating techniques.[“Everything You Need to Know About Lost Wax Casting”. www.deangroup-int.co.uk. Retrieved 2021-10-27.] The earliest known text that describes the investment casting process (Schedula Diversarum Artium) was written around 1100 A.D. by Theophilus Presbyter, a monk who described various manufacturing processes, including the recipe for parchment. This book was used by sculptor and goldsmith Benvenuto Cellini (1500–1571), who detailed in his autobiography the investment casting process he used for the Perseus with the Head of Medusa sculpture that stands in the Loggia dei Lanzi in Florence, Italy. Investment casting came into use as a modern industrial process in the late 19th century, when dentists began using it to make crowns and inlays, as described by Barnabas Frederick Philbrook of Council Bluffs, Iowa in 1897.[Asgar K (1988). “Casting Metals in Dentistry: Past – Present – Future” (PDF). Advances in Dental Research. 1 (2): 33–43. doi:10.1177/08959374880020011701. hdl:2027.42/67759. PMID 3073783. S2CID 17215227.] Its use was accelerated by William H. Taggart of Chicago, whose 1907 paper described his development of a technique[citation needed]. He also formulated a wax pattern compound of excellent properties, developed an investment material, and invented an air-pressure casting machine. In the 1940s, World War II increased the demand for precision net shape manufacturing and specialized alloys that could not be shaped by traditional methods, or that required too much machining. Industry turned to investment casting. After the war, its use spread to many commercial and industrial applications that used complex metal parts.
- Bibliography and External links
- American Society for Metals; ASM International Handbook Committee; ASM International Alloy Phase Diagram Committee (1990), ASM Handbook: Casting, vol. 15 (10th ed.), ASM International, ISBN 978-0-87170-021-6.
- Degarmo, E. Paul; Black, J T.; Kohser, Ronald A. (2003), Materials and Processes in Manufacturing (9th ed.), Wiley, ISBN 0-471-65653-4.
- Sias, Fred R. (2006), Lost-wax Casting: Old, New, and Inexpensive Methods (illustrated ed.), Woodsmere Press, ISBN 978-0-9679600-0-5.
- Kalpakjian, Serope; Schmid, Steven (2006), Manufacturing Engineering and Technology (5th ed.), Pearson, ISBN 0-13-148965-8.
- Wikimedia Commons has media related to Investment casting.
- “The Spectrum of Investment Casting Possibilities: PMI Alloy and Engineering Guide” (PDF). Precision Metalsmiths, Inc. Archived from the original (PDF) on 2012-02-19. Retrieved 2009-02-23.
- Kevin Michaels (Feb 7, 2019). “Opinion: Why The Supply Chain’s Achilles’ Heel Is 5,000 Years Old”. Aviation Week & Space Technology.
- See also
- Full-mold casting
- Full-mold casting is an evaporative-pattern casting process which is a combination of sand casting and lost-foam casting. It uses an expanded polystyrene foam pattern which is then surrounded by sand, much like sand casting. The metal is then poured directly into the mold, which vaporizes the foam upon contact. PROCESS – First, a pattern is usually made from polystyrene foam, which can be done many different ways. For small volume runs the pattern can be hand cut or machined from a solid block of foam; if the geometry is simple enough it can even be cut using a hot-wire foam cutter. If the volume is large, then the pattern can be mass-produced by a process similar to injection molding. Pre-expanded beads of polystyrene are injected into a preheated aluminum mold at low pressure. Steam is then applied to the polystyrene which causes it to expand more to fill the die. The final pattern is approximately 97.5% air and 2.5% polystyrene. The finished patterns can be hot glued to pre-made pouring basins, runners, and risers to form the final pattern.[Degarmo, E. Paul; Black, J T.; Kohser, Ronald A. (2003), Materials and Processes in Manufacturing (9th ed.), Wiley, ISBN 0-471-65653-4.] The pattern is then coated with a refractory material. The coated pattern (2) is placed in a flask and packed carefully with green sand (4) or a chemically bonded sand. Finally, the molten metal (1) is poured into the mold, which vaporizes the foam (3) allowing the metal to fill the entire mold. The vapor is simultaneously extracted from the flask through the sand. The casting is allowed to cool and then dumped out of the flask (5) ready to use. The sand does not need to be reprocessed so it can be directly reused.[Degarmo, E. Paul; Black, J T.; Kohser, Ronald A. (2003), Materials and Processes in Manufacturing (9th ed.), Wiley, ISBN 0-471-65653-4.][Kalpakjian, Serope; Schmid, Steven (2006), Manufacturing Engineering and Technology (5th ed.), Pearson, ISBN 0-13-148965-8.] DETAILS – The minimum wall thickness for a full-mold casting is 2.5 mm (0.10 in). Typical dimensional tolerances are 0.3% and typical surface finishes are from 2.5 to 25 µm (100 to 1000 µin) RMS. The size range is from 400 g (0.88 lb) to several tonnes (tons).[Full Mold Casting, retrieved 2009-03-24.] Full-mold casting is often used to produce cylinder heads, engine blocks, pump housings, automotive brake components, and manifolds.[Full Mold Casting, retrieved 2009-03-24.] Commonly employed materials include aluminium, iron, steel, nickel alloys, and copper alloys.[Kalpakjian, Serope; Schmid, Steven (2006), Manufacturing Engineering and Technology (5th ed.), Pearson, ISBN 0-13-148965-8.] ADVANTAGES AND DISADVANTAGES – This casting process is advantageous for very complex castings, that would regularly require cores. It is also dimensionally accurate, requires no draft, and has no parting lines so no flash is formed. As compared to investment casting, it is cheaper because it is a simpler process and the foam is cheaper than the wax. Risers are not usually required due to the nature of the process; because the molten metal vaporizes the foam the first metal into the mold cools more quickly than the rest, which results in natural directional solidification.[Degarmo, E. Paul; Black, J T.; Kohser, Ronald A. (2003), Materials and Processes in Manufacturing (9th ed.), Wiley, ISBN 0-471-65653-4.][Kalpakjian, Serope; Schmid, Steven (2006), Manufacturing Engineering and Technology (5th ed.), Pearson, ISBN 0-13-148965-8.] The two main disadvantages are that pattern costs can be high for low volume applications and the patterns are easily damaged or distorted due to their low strength.[Degarmo, E. Paul; Black, J T.; Kohser, Ronald A. (2003), Materials and Processes in Manufacturing (9th ed.), Wiley, ISBN 0-471-65653-4.] If a die is used to create the patterns there is a large initial cost.[Kalpakjian, Serope; Schmid, Steven (2006), Manufacturing Engineering and Technology (5th ed.), Pearson, ISBN 0-13-148965-8.] HISTORY – Main article: Evaporative-pattern casting. The first patent for an evaporative-pattern casting process was filed in April 1956, by H.F. Shroyer. He patented the use of foam patterns embedded in traditional green sand for metal casting.
- Lost-foam casting
- Lost-foam casting (LFC) is a type of evaporative-pattern casting process that is similar to investment casting except foam is used for the pattern instead of wax. This process takes advantage of the low boiling point of polymer foams to simplify the investment casting process by removing the need to melt the wax out of the mold. PROCESS – First, a pattern is made from polystyrene foam, which can be done by many different ways. For small volume runs the pattern can be hand cut or machined from a solid block of foam, or a sheet of foam core board if the geometry is simple enough it can even be cut using a hot-wire foam cutter. If the volume is large, then the pattern can be mass-produced by a process similar to injection molding. Pre-expanded beads of polystyrene are injected into a preheated aluminum mold at low pressure. Steam is then applied to the polystyrene which causes it to expand more to fill the die. The final pattern is approximately 97.5% air and 2.5% polystyrene. Pre-made pouring basins, runners, and risers can be hot glued to the pattern to finish it.[Degarmo, E. Paul; Black, J T.; Kohser, Ronald A. (2003), Materials and Processes in Manufacturing (9th ed.), Wiley, ISBN 0-471-65653-4.] The foam pattern does not need to be coated with investment if high detail is not needed, simply putting the foam pattern in a box, filling with sand and vibrating will do. However, when detail is needed, the foam cluster is coated with ceramic investment, also known as the refractory coating, via dipping, brushing, spraying or flow coating. After the coating dries, the cluster is placed into a flask and backed up with un-bonded sand which is compacted using a vibration table. The refractory coating captures all of the detail in the foam model and creates a barrier between the smooth foam surface and the coarse sand surface. Secondly it controls permeability, which allows the gas created by the vaporized foam pattern to escape through the coating and into the sand. Controlling permeability is a crucial step to avoid sand erosion. Finally, it forms a barrier so that molten metal does not penetrate or cause sand erosion during pouring. [Degarmo, E. Paul; Black, J T.; Kohser, Ronald A. (2003), Materials and Processes in Manufacturing (9th ed.), Wiley, ISBN 0-471-65653-4.][ASM (2002), “Lost Foam Casting”, ASM Handbook Volume 15, Casting.] Once the sand is compacted, the mold is ready to be poured. Automatic pouring is commonly used in LFC, as the pouring process is significantly more critical than in conventional foundry practice.[citation needed] There is no bake-out phase, as for lost-wax. The melt is poured directly into the foam-filled mold, burning out the foam as it pours. As the foam is of low density, the waste gas produced by this is relatively small and can escape through mold permeability, as for the usual outgassing control. DETAILS – Commonly cast metals include cast irons, aluminium alloys, steels, and nickel alloys; less frequently stainless steels and copper alloys are also cast. The size range is from 0.5 kg (1.1 lb) to several tonnes (tons). The minimum wall thickness is 2.5 mm (0.098 in)[citation needed] and there is no upper limit. Typical surface finishes are from 2.5 to 25 µm (100 to 1000 µin) RMS.[Degarmo, E. Paul; Black, J T.; Kohser, Ronald A. (2003), Materials and Processes in Manufacturing (9th ed.), Wiley, ISBN 0-471-65653-4.] Typical linear tolerances are ±0.127 mm/mm (0.005 in/in).[Top ten lost foam casting questions, retrieved 2009-03-29.] ADVANTAGES AND DISADVANTAGES – This casting process is advantageous for very complex castings that would regularly require cores. It is also dimensionally accurate, maintains an excellent surface finish, requires no draft, and has no parting lines so no flash is formed. The un-bonded sand of lost foam casting can be much simpler to maintain than green sand and resin bonded sand systems. Lost foam is generally more economical than investment casting because it involves fewer steps. Risers are not usually required due to the nature of the process; because the molten metal vaporizes the foam the first metal into the mold cools more quickly than the rest, which results in natural directional solidification.[Degarmo, E. Paul; Black, J T.; Kohser, Ronald A. (2003), Materials and Processes in Manufacturing (9th ed.), Wiley, ISBN 0-471-65653-4.][Kalpakjian, Serope; Schmid, Steven (2006), Manufacturing Engineering and Technology (5th ed.), Pearson, ISBN 0-13-148965-8.] Foam is easy to manipulate, carve and glue, due to its unique properties. The flexibility of LFC often allows for consolidating the parts into one integral component; other forming processes would require the production of one or more parts to be assembled.[Degarmo, E. Paul; Black, J T.; Kohser, Ronald A. (2003), Materials and Processes in Manufacturing (9th ed.), Wiley, ISBN 0-471-65653-4.]HISTORY Main article: Evaporative-pattern casting. Lost-foam casting was invented in the early fifties by Canadian sculptor Armand Vaillancourt. Public recognition of the benefits of LFC was made by General Motors in the mid 1980s when it announced its new car line, Saturn, would utilize LFC for production of all engine blocks, cylinder heads, crankshafts, differential carriers, and transmission cases.[Spada, Alfred T. (December 2001), “GM Unveils Latest Lost Foam Success: Setting the bar for lost foam casters, GM’s newest venture at Saginaw Metal Casting Operations has ramped up in record time to produce cylinder heads and blocks for the firm’s award-winning SUVs”, Modern Casting: 1.]
- Full-mold casting
CONSUMER GOODS – PLA is used in a large variety of consumer products such as disposable tableware, cutlery, housings for kitchen appliances and electronics such as laptops and handheld devices, and microwavable trays. (However, PLA is not suitable for microwavable containers because of its low glass transition temperature.) It is used for compost bags, food packaging and loose-fill packaging material that is cast, injection molded, or spun.[Auras R, Lim LT, Selke SE, Tsuji H, eds. (2010). Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications. doi:10.1002/9780470649848. ISBN 978-0-470-29366-9.] In the form of a film, it shrinks upon heating, allowing it to be used in shrink tunnels. In the form of fibers, it is used for monofilament fishing line and netting. In the form of nonwoven fabrics, it is used for upholstery, disposable garments, awnings, feminine hygiene products, and diapers. PLA has applications in engineering plastics, where the stereocomplex is blended with a rubber-like polymer such as ABS. Such blends have good form stability and visual transparency, making them useful in low-end packaging applications. PLA is used for automotive parts such as floor mats, panels, and covers. Its heat resistance and durability are inferior to the widely used polypropylene (PP), but its properties are improved by means such as capping of the end groups to reduce hydrolysis.[Auras R, Lim LT, Selke SE, Tsuji H, eds. (2010). Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications. doi:10.1002/9780470649848. ISBN 978-0-470-29366-9.]
AGRICULTURAL – In the form of fibers, PLA is used for monofilament fishing line and netting for vegetation and weed prevention. It is used for sandbags, planting pots, binding tape and ropes .[Auras R, Lim LT, Selke SE, Tsuji H, eds. (2010). Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications. doi:10.1002/9780470649848. ISBN 978-0-470-29366-9.]
MEDICAL – PLA can degrade into innocuous lactic acid, making it suitable for use as medical implants in the form of anchors, screws, plates, pins, rods, and mesh.[Auras R, Lim LT, Selke SE, Tsuji H, eds. (2010). Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications. doi:10.1002/9780470649848. ISBN 978-0-470-29366-9.] Depending on the type used, it breaks down inside the body within 6 months to 2 years. This gradual degradation is desirable for a support structure, because it gradually transfers the load to the body (e.g., to the bone) as that area heals. The strength characteristics of PLA and PLLA implants are well documented.[Nazre A, Lin S (1994). Harvey JP, Games RF (eds.). Theoretical Strength Comparison of Bioabsorbable (PLLA) Plates and Conventional Stainless Steel and Titanium Plates Used in Internal Fracture Fixation. p. 53. ISBN 978-0-8031-1897-3.] Thanks to its bio-compatibility and biodegradability, PLA found interest as a polymeric scaffold for drug delivery purposes. The composite blend of poly(L-lactide-co-D,L-lactide) (PLDLLA) with tricalcium phosphate (TCP) is used as PLDLLA/TCP scaffolds for bone engineering.[Lam CX, Olkowski R, Swieszkowski W, Tan KC, Gibson I, Hutmacher DW (2008). “Mechanical and in vitro evaluations of composite PLDLLA/TCP scaffolds for bone engineering”. Virtual and Physical Prototyping. 3 (4): 193–197. doi:10.1080/17452750802551298. S2CID 135582844][Bose S, Vahabzadeh S, Bandyopadhyay A (2013). “Bone tissue engineering using 3D printing”. Materials Today. 16 (12): 496–504. doi:10.1016/j.mattod.2013.11.017.] Poly-L-lactic acid (PLLA) is the main ingredient in Sculptra, a facial volume enhancer used for treating lipoatrophy of the cheeks. PLLA is used to stimulate collagen synthesis in fibroblasts via foreign body reaction in the presence of macrophages. Macrophages act as a stimulant in secretion of cytokines and mediators such as TGF-β, which stimulate the fibroblast to secrete collagen into the surrounding tissue. Therefore, PLLA has potential applications in the dermatological studies.[ Ray S, Adelnia H, Ta HT (September 2021). “Collagen and the effect of poly-l-lactic acid based materials on its synthesis”. Biomaterials Science. 9 (17): 5714–5731. doi:10.1039/d1bm00516b. hdl:10072/405917. PMID 34296717. S2CID 236199608.][Ray S, Ta HT (July 2020). “Investigating the Effect of Biomaterials Such as Poly-(l-Lactic Acid) Particles on Collagen Synthesis In Vitro: Method Is Matter”. Journal of Functional Biomaterials. 11 (3): 51. doi:10.3390/jfb11030051. PMC 7564527. PMID 32722074.] PLLA is under investigation as a scaffold that can generate a small amount of electric current via the piezoelectric effect that stimulates the growth of mechanically robust cartilage in multiple animal models.[Petersen M (18 January 2022). “Electric knee implants could help millions of arthritis patients”. ZME Science. Retrieved 19 January 2022.]
DEGRADATION – PLA is degraded abiotically by three mechanisms:[Castro-Aguirre E, Iñiguez-Franco F, Samsudin H, Fang X, Auras R (December 2016). “Poly(lactic acid)-Mass production, processing, industrial applications, and end of life”. Advanced Drug Delivery Reviews. 107: 333–366. doi:10.1016/j.addr.2016.03.010. PMID 27046295. ^]
- Hydrolysis: The ester groups of the main chain are cleaved, thus reducing molecular weight.
- Thermal decomposition: A complex phenomenon leading to the appearance of different compounds such as lighter molecules and linear and cyclic oligomers with different Mw, and lactide.
- Photodegradation: UV radiation induces degradation. This is a factor mainly where PLA is exposed to sunlight in its applications in plasticulture, packaging containers and films.
The hydrolytic reaction is: −COO+H2O⟶−COOH+−OH−
The degradation rate is very slow in ambient temperatures. A 2017 study found that at 25 °C (77 °F) in seawater, PLA showed no loss of mass over a year, but the study did not measure breakdown of the polymer chains or water absorption.[Bagheri AR, Laforsch C, Greiner A, Agarwal S (July 2017). “Fate of So-Called Biodegradable Polymers in Seawater and Freshwater”. Global Challenges. 1 (4): 1700048. doi:10.1002/gch2.201700048. PMC 6607129. PMID 31565274.] As a result, it degrades poorly in landfills and household composts, but is effectively digested in hotter industrial composts, usually degrading best at temperatures of over 60 °C (140 °F).[“Is PLA Biodegradable? – The Truth”. All3DP. 10 December 2019. Retrieved 26 June 2021.] Pure PLA foams are selectively hydrolysed in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with fetal bovine serum (FBS) (a solution mimicking body fluid). After 30 days of submersion in DMEM+FBS, a PLLA scaffold lost about 20% of its weight.[Pavia FC, La Carrubba V, Piccarolo S, Brucato V (August 2008). “Polymeric scaffolds prepared via thermally induced phase separation: tuning of structure and morphology”. Journal of Biomedical Materials Research. Part A. 86 (2): 459–466. doi:10.1002/jbm.a.31621. PMID 17975822.] PLA samples of various molecular weights were degraded into methyl lactate (a green solvent) by using a metal complex catalyst.[Román-Ramírez LA, Mckeown P, Jones MD, Wood J (4 January 2019). “Poly(lactic acid) Degradation into Methyl Lactate Catalyzed by a Well-Defined Zn(II) Complex”. ACS Catalysis. 9 (1): 409–416. doi:10.1021/acscatal.8b04863.][McKeown P, Román-Ramírez LA, Bates S, Wood J, Jones MD (November 2019). “Zinc Complexes for PLA Formation and Chemical Recycling: Towards a Circular Economy”. ChemSusChem. 12 (24): 5233–5238. doi:10.1002/cssc.201902755. PMID 31714680. S2CID 207941305.][Román-Ramírez LA, McKeown P, Shah C, Abraham J, Jones MD, Wood J (June 2020). “Chemical Degradation of End-of-Life Poly(lactic acid) into Methyl Lactate by a Zn(II) Complex”. Industrial & Engineering Chemistry Research. 59 (24): 11149–11156. doi:10.1021/acs.iecr.0c01122. PMC 7304880. PMID 32581423.] PLA can also be degraded by some bacteria, such as Amycolatopsis and Saccharothrix. A purified protease from Amycolatopsis sp., PLA depolymerase, can also degrade PLA. Enzymes such as pronase and most effectively proteinase K from Tritirachium album degrade PLA.[Tokiwa Y, Calabia BP, Ugwu CU, Aiba S (August 2009). “Biodegradability of plastics”. International Journal of Molecular Sciences. 10 (9): 3722–3742. doi:10.3390/ijms10093722. PMC 2769161. PMID 19865515.]

END OF LIFE – Four possible end-of-life scenarios are the most common:
- Recycling:[Dash, Aparna; Kabra, Shruti; Misra, Sidhant; G, Hrishikeshan; Singh, Raghvendra Pratap; Patterson, Albert E; Chadha, Utkarsh; Rajan, A John; Hirpha, Bulcha Bekele (1 November 2022). “Comparative property analysis of fused filament fabrication PLA using fresh and recycled feedstocks”. Materials Research Express. 9 (11): 115303. doi:10.1088/2053-1591/ac96d4. S2CID 252665567.] which can be either chemical or mechanical. Currently, the SPI resin identification code 7 (“others”) is applicable for PLA. In Belgium, Galactic started the first pilot unit to chemically recycle PLA (Loopla).[“Chemical recycling closes the LOOPLA for cradle-to-cradle PLA”. 20 November 2015.] Unlike mechanical recycling, waste material can hold various contaminants. Polylactic acid can be chemically recycled to monomer by thermal depolymerization or hydrolysis. When purified, the monomer can be used for the manufacturing of virgin PLA with no loss of original properties [Gorrasi G, Pantani R (2017). “Hydrolysis and Biodegradation of Poly(lactic acid)”. In Di Lorenzo ML, Androsch R (eds.). Synthesis, Structure and Properties of Poly(lactic acid). Advances in Polymer Science. Vol. 279. Cham: Springer International Publishing. pp. 119–151. doi:10.1007/12_2016_12. ISBN 978-3-319-64229-1.] (cradle-to-cradle recycling).[dubious – discuss] End-of-life PLA can be chemically recycled to methyl lactate by transesterification.[Román-Ramírez LA, McKeown P, Shah C, Abraham J, Jones MD, Wood J (June 2020). “Chemical Degradation of End-of-Life Poly(lactic acid) into Methyl Lactate by a Zn(II) Complex”. Industrial & Engineering Chemistry Research. 59 (24): 11149–11156. doi:10.1021/acs.iecr.0c01122. PMC 7304880. PMID 32581423.]
- Composting: PLA is biodegradable under industrial composting conditions, starting with chemical hydrolysis process, followed by microbial digestion, to ultimately degrade the PLA. Under industrial composting conditions (58 °C (136 °F)), PLA can partly (about half) decompose into water and carbon dioxide in 60 days, after which the remainder decomposes much more slowly,[Iovino R, Zullo R, Rao MA, Cassar L, Gianfreda L (2008). “Biodegradation of poly(lactic acid)/starch/coir biocompositesunder controlled composting conditions”. Polymer Degradation and Stabilit. 93: 147. doi:10.1016/j.polymdegradstab.2007.10.011.] with the rate depending on the material’s degree of crystallinity.[Pantani R, Sorrentino A (2013). “Influence of crystallinity on the biodegradation rate of injection-moulded poly(lactic acid) samples in controlled composting conditions”. Polymer Degradation and Stability. 98 (5): 1089. doi:10.1016/j.polymdegradstab.2013.01.005.] Environments without the necessary conditions will see very slow decomposition akin to that of non-bioplastics, not fully decomposing for hundreds or thousands of years.[“How long does it take for plastics to biodegrade?”. HowStuffWorks. 15 December 2010. Retrieved 9 March 2021.]
- Incineration: PLA can be incinerated without producing chlorine-containing chemicals or heavy metals because it contains only carbon, oxygen, and hydrogen atoms. Since it does not contain chlorine it does not produce dioxins or hydrochloric acid during incineration.[“End of Life Options for Bioplastics – Recycling, Energy, Composting, Landfill – Bioplastics Guide | Bioplastics Guide”. Retrieved 9 March 2021. ^] PLA can be combusted with no remaining residue. This and other results suggest that incineration is an environmentally friendly disposal of waste PLA.[Chien YC, Liang C, Liu SH, Yang SH (July 2010). “Combustion kinetics and emission characteristics of polycyclic aromatic hydrocarbons from polylactic acid combustion”. Journal of the Air & Waste Management Association. 60 (7): 849–855. doi:10.3155/1047-3289.60.7.849. PMID 20681432. S2CID 34100178.]
- Landfill: the least preferable option is landfilling because PLA degrades very slowly in ambient temperatures, often as slowly as other plastics.[“How long does it take for plastics to biodegrade?”. HowStuffWorks. 15 December 2010. Retrieved 9 March 2021.]
See also
- Acrylonitrile butadiene styrene (ABS) – also used for 3D printing
- Cellophane, polyglycolide, plastarch material, poly-3-hydroxybutyrate – biologically derived polymers
- Polilactofate
- Polycaprolactone
- Zein, shellac – biologically derived coating materials
- Poly(methyl methacrylate)
References
- Hakimi O, Knight DP, Vollrath F, Vadgama P (April 2007). “Spider and mulberry silkworm silks as compatible biomaterials”. Composites Part B: Engineering. 38 (3): 324–37. doi:10.1016/j.compositesb.2006.06.012.
- Dyakonov T, Yang CH, Bush D, Gosangari S, Majuru S, Fatmi A (2012). “Design and characterization of a silk-fibroin-based drug delivery platform using naproxen as a model drug”. Journal of Drug Delivery. 2012: 490514. doi:10.1155/2012/490514. PMC 3312329. PMID 22506122.
- “Brin definition and meaning | Collins English Dictionary”. www.collinsdictionary.com.
- Inoue S, Tanaka K, Arisaka F, Kimura S, Ohtomo K, Mizuno S (December 2000). “Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio”. The Journal of Biological Chemistry. 275 (51): 40517–28. doi:10.1074/jbc.M006897200. PMID 10986287.
- Valluzzi R, Gido SP, Muller W, Kaplan DL (1999). “Orientation of silk III at the air-water interface”. International Journal of Biological Macromolecules. 24 (2–3): 237–42. doi:10.1016/S0141-8130(99)00002-1. PMID 10342770.
- Tokiwa Y, Calabia BP, Ugwu CU, Aiba S (August 2009). “Biodegradability of plastics”. International Journal of Molecular Sciences. 10 (9): 3722–42. doi:10.3390/ijms10093722. PMC 2769161. PMID 19865515.
This article incorporates text from the public domain Pfam and InterPro: IPR009911



Leave a Reply