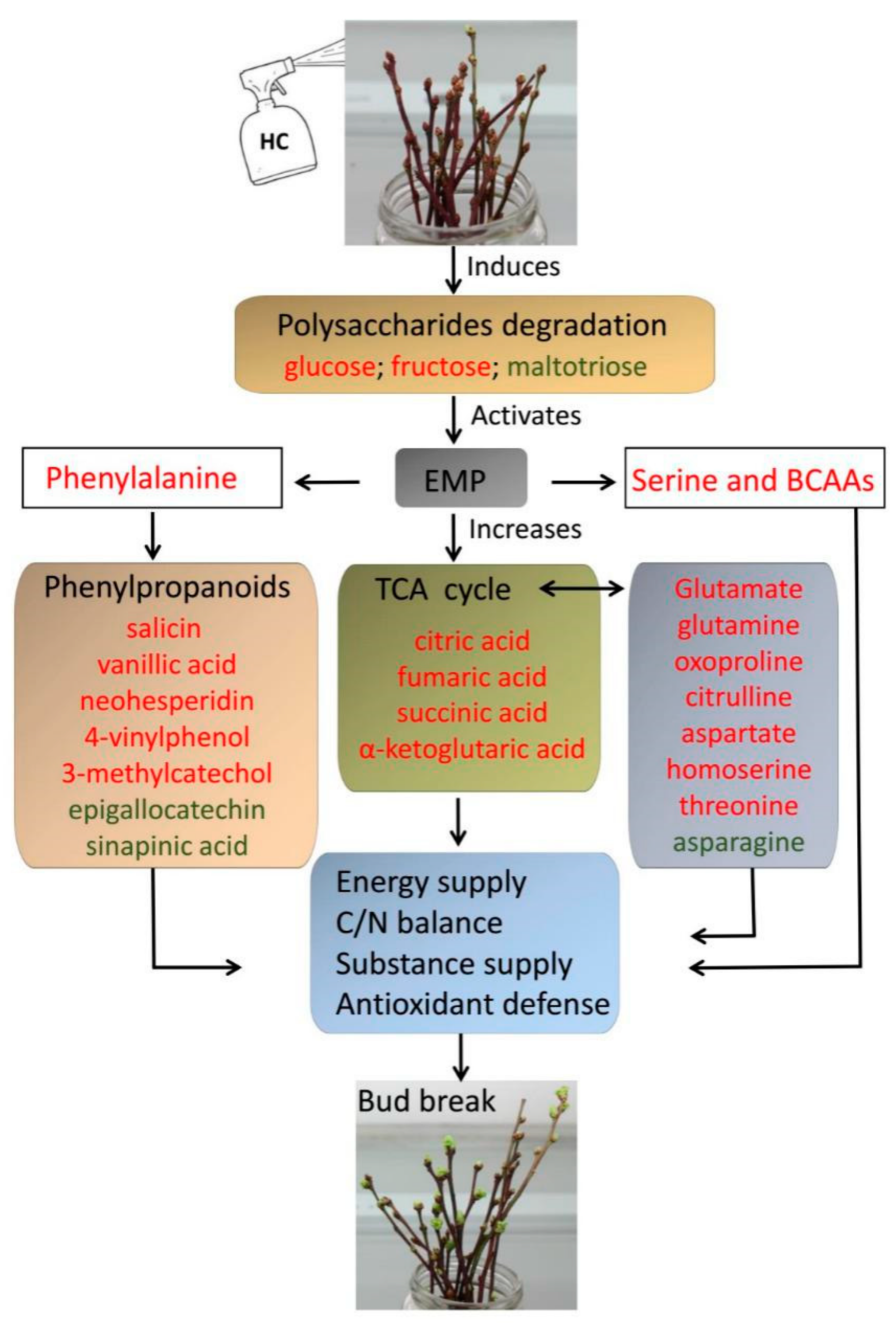
Cyanamide notes (it was a polio vaccine that spurred these notes and by now polio has five mentions on the page and these are two of them)
I’m going to add some polio vaccine stuff at the top of these notes. Hilary Koprowski is the one mentioned on the Polio Hall of Fame page who was not included in the hideous monument, see What In God’s Name, even though he (and his work) have direct connection to those who are included. He did a chunk of his work at something called Lederle Laboratories, the pharmaceutical division of American Cyanamid. This is what Wikipedia has to say about him.

Hilary Koprowski (1916–2013) was a Polish virologist and immunologist active in the United States who demonstrated the world’s first effective live polio vaccine. He authored or co-authored over 875 scientific papers and co-edited several scientific journals. Koprowski received many academic honors and national decorations, including the Belgian Order of the Lion, the French Order of Merit and Legion of Honour, Finland’s Order of the Lion, and the Order of Merit of the Republic of Poland.
Koprowski was the target of accusations in the press related to the “oral polio vaccine AIDS hypothesis“, which posited that the AIDS pandemic originated from live polio vaccines such as Koprowski’s. This allegation has long been refuted by evidence showing that the human immunodeficiency virus was introduced to humans before his polio-vaccine trials were conducted in Africa. The case was settled out of court with a formal apology from Rolling Stone magazine.
- Worobey M, Santiago ML, Keele BF, Ndjango JB, Joy JB, Labama BL, Dhed’A BD, Rambaut A, Sharp PM, Shaw GM, Hahn BH (2004). “Origin of AIDS: contaminated polio vaccine theory refuted” (PDF file, direct download 203 KB). Nature. 428 (6985): 820. Bibcode:2004Natur.428..820W. doi:10.1038/428820a. PMID 15103367. S2CID 4418410.
Note 1,13: Korber, B.et al. Science 288, 1789–1796 (2000)
- Martin B (August 2003). “Investigating the origin of AIDS: some ethical dimensions”. J Med Ethics. 29 (4): 253–256. doi:10.1136/jme.29.4.253. PMC 1733782. PMID 12930866.
In 1939, after Germany‘s invasion of Poland, Koprowski and his wife, likewise a physician, fled the country, using Koprowski family business connections in Manchester, England. Hilary went to Rome, where he spent a year studying piano at the Santa Cecilia Conservatory; while Irena went to France, where she gave birth to their first child, Claude Koprowski, and worked as an attending physician at a psychiatric hospital.
- Koprowska, Irena (1997). A Woman Wanders Through Life and Science. State University of New York Press. ISBN 9780791431771.
As the invasion of France loomed in 1940, Irena and the infant escaped from France via Spain and Portugal —where the Koprowski family reunited — to Brazil, where Koprowski worked in Rio de Janeiro for the Rockefeller Foundation. His field of research for several years was finding a live-virus vaccine against yellow fever. After World War II the Koprowskis settled in Pearl River, New York, where Hilary was hired as a researcher for Lederle Laboratories, the pharmaceutical division of American Cyanamid. Here he began his polio experiments, which ultimately led to the creation of the first oral polio vaccine. Koprowski served as director of the Wistar Institute, 1957–91, during which period Wistar achieved international recognition for its vaccine research and became a National Cancer Institute Cancer Center.[citation needed]
While at Lederle Laboratories, Koprowski created an early polio vaccine, based on an orally administered attenuated polio virus. In researching a potential polio vaccine, he had focused on live viruses that were attenuated (rendered non-virulent) rather than on killed viruses (the latter became the basis for the injected vaccine subsequently developed by Jonas Salk).
- Hilary Koprowski, Who Developed First Live-Virus Polio Vaccine, Dies at 96 – The New York Times, April 20, 2013.
Koprowski viewed the live vaccine as more powerful, since it entered the intestinal tract directly and could provide lifelong immunity, whereas the Salk vaccine required booster shots. Also, administering a vaccine by mouth is easy, whereas an injection requires medical facilities and is more expensive.
- Zheng, Zhichao; Diaz-Arévalo, Diana; Guan, Hongbing; Zeng, Mingtao (2018-07-03). “Noninvasive vaccination against infectious diseases”. Human Vaccines & Immunotherapeutics. 14 (7): 1717–1733. doi:10.1080/21645515.2018.1461296. ISSN 2164-5515. PMC 6067898. PMID 29624470.
Koprowski developed his polio vaccine by attenuating the virus in brain cells of a cotton rat, Sigmodon hispidus, a New World species that is susceptible to polio. He administered the vaccine to himself in January 1948 and, on 27 February 1950, to 20 children at Letchworth Village, a home for disabled persons in Rockland County, New York. Seventeen of the 20 children developed antibodies to polio virus — the other three apparently already had antibodies — and none of the children developed complications. Within 10 years, the vaccine was being used on four continents.
- Hilary Koprowski, Who Developed First Live-Virus Polio Vaccine, Dies at 96 – The New York Times, April 20, 2013.
A few words about Letchworth Village, Irving Haberman, Robert F. Kennedy and Geraldo Rivera
Letchworth Village was a residential institution located in Rockland County, New York, in the hamlet of Thiells built for the physically and mentally disabled of all ages, from the newborn to the elderly. Opened in 1911, Letchworth Village at its peak consisted of over 130 buildings spread out over many acres of land. It was named for William Pryor Letchworth, who espoused reform in the treatment and care of the insane, epileptics, and poor children.
By the end of 1911, the first phase of construction had completed on the 2,362-acre “state institution for the segregation of the epileptic and feeble-minded.” With architecture modeled after Monticello, the picturesque community was lauded as a model institution for the treatment of the developmentally disabled, a humane alternative to high-rise asylums, having been founded on several guiding principles that were revolutionary at the time. Separate living and training facilities for children, able-bodied adults, and the infirm were not to exceed two stories or house over 70 inmates. Until the 1960s, the able-bodied labored on communal farms, raising enough food and livestock to feed the entire population.
- AbandonedNYC (6 August 2012). “Legend Tripping in Letchworth Village”. Retrieved 4 December 2012.
It was conceived by the progressives of the time as a major departure from the almshouses of the 19th century. The facility was thought to have had great potential and was a great improvement from past facilities. It was a farming village of nearly four square miles, In the words of the 1927 Rockland County Red Book, “subdivided as far as possible in order to avoid the tendency toward institutionalism.”
Letchworth was described as an ideal center for the mentally challenged and praised by the state at first. Yet rumors such as the mistreatment of patients and horrific experimenting continued to circulate long after its closing. Former worker Dr. Little presented in an annual report in 1921 that there were three categories of “feeble-mindedness”: the “moron” group, the “imbecile” group, and the “idiot” group. The last of these categories is the one that could not be trained, Dr. Little said, and so they should not be taken into Letchworth Village, because they were unable to “benefit the state” by doing the various jobs that were assigned to the male patients, included loading thousands of tons of coal into storage facilities, building roads, and farming acres of land.
Many of the patients were young children. In 1921, the 13th Annual Report lists the number of patients admitted that year. Out of 506 people, 317 were between the ages of 5 and 16, and 11 were under the age of 5 years. Visitors observed that the children were malnourished and looked sick. The Letchworth staff claimed in the report that there was a scarcity of food, water, and other necessary supplies, but that was not the case. Children were often the subjects of testing and some of the cruelest neglect. Many of the children were able to comprehend learning but were not given the chance because they were thought of as “different.” By the 1950s, the Village was overflowing with 4,000 inhabitants.
Buteux, Lindsay. “Letchworth: The Village of Secrets”. Student Outlook Press. Retrieved 4 December 2012.
In the 1940s, Irving Haberman did a set of photographs which revealed the true nature of what was going on. Until this point, the conditions of the facility weren’t apparent to the public. Haberman’s photos exposed the terrible conditions of the facilities as well as the dirty, unkempt patients. Naked residents huddled in sterile day rooms. The photos showed the patients to be highly neglected. These photos pushed the public to question the institution and demand answers. Haberman knew that these photos would bring attention to the Letchworth facility.
- Trent, James W. (1994). Inventing the Feeble Mind: A History of Mental Retardation in the United States. University of California Press. p. 226.
Looks like they got the attention of a few people…In February 1950, while Letchworth still enjoyed a good reputation (what?) amongst health professionals (despite rumors of overcrowding and maltreatment), Letchworth’s Dr. George Jervis asked Dr. Hilary Koprowski to test his live-virus polio vaccine at Letchworth Village to compare it to the alternatives available then. Koprowski viewed these experiments as a positive first step toward a better polio vaccine…I’m not saying he is an off the boat nazi, even though he certainly looks like one, but they do have a history of doing just that kind of thing in just those kinds of places and in just that kind of ridiculous order. And who else would go spend a year playing piano in Rome while they are throwing a war? How were they escaping to nearly every place on earth (and right ahead of the nazis?) with all hell breaking loose? There is some bullshit afoot here for sure but I’m a little creeped out and it’s not really my focus. It sure does keep coming up, however.
- Oshinsky, David M. (2005). Polio:An American Story: An American Story. Oxford University Press. p. 136.
- “A look at Letchworth Village: Meet the people who influenced the institution”.
In 1972, ABC News featured Letchworth Village in its piece “Willowbrook: The Last Great Disgrace”. The documentary, by ABC New York’s investigative reporter Geraldo Rivera, looked at how intellectually disabled people, particularly children, were being treated in the State of New York. United States Senator Robert F. Kennedy previously had toured the Willowbrook facility in 1965 and called it a “snake pit.” Kennedy was not allowed to take cameras into the buildings, however, so the average citizen had no idea how bad the conditions inside Willowbrook actually were. Kennedy’s speeches about the conditions there, although impassioned, attracted little attention and resulted in little or no improvement in conditions at the facility.
Rivera, on the other hand, arrived at Willowbrook with a full camera crew, and when the documentary was aired, there was widespread outrage at how the residents at Willowbrook, many of them children, were being mistreated. Although Rivera’s documentary focused on the Willowbrook State School on Staten Island, Rivera also visited Letchworth Village, as well as facilities in California. While he found that a great deal of progress had been made in the caring for, and training of, disabled people in California, he saw the situation in New York’s facilities as backward and cruel.
Rivera accompanied Bronx congressman Mario Biaggi to Letchworth Village, arriving two hours early because Rivera correctly suspected that the staff would be ordered to clean and dress the children before the camera crew arrived. Biaggi described the children there as being subjected to “[t}he worst possible conditions I’ve ever seen in my life”.
The documentary showed the residents of Willowbrook and Letchworth Village, many of them children, living in awful, dirty and overcrowded conditions, with a lack of clothing, bathing, and attention to their most basic needs. The facilities were incredibly understaffed, and there was little or no actual schooling, training, or even simple activities to keep residents occupied.
Rivera saw the overcrowding and neglect as a direct result of inadequate funding and the ignorant attitudes in wider society. The potential of individual patients was far from being realized. This confronting report helped lead to far-reaching reform of disability services throughout the United States.
- “Geraldo Rivera: Willowbrook”.
- Rivera, Geraldo (1972). Willowbrook: The Last Great Disgrace. WABC-TV.
Letchworth was closed in 1996, leaving the buildings to decay. Many who worked at the Village refuse to speak of their experiences. Old methods of segregating patients and the disabled were changed to including them in society and bringing a normalization to them. Patients were moved to more up-to-date facilities in the county.
- Buteux, Lindsay. “Letchworth: The Village of Secrets”. Student Outlook Press. Retrieved 4 December 2012.

- History and photos of the abandoned campus.
- History, present day photos, and video of the Letchworth Village grounds.
- Aerial drone video of the exterior of the hospital building at Letchworth Village, taken in June 2021.
- Aerial drone video of the exterior of the Stewart Hall Boys’ Dormitory at Letchworth Village, taken in July 2021.
- Drone video of the Old Letchworth Village Cemetery, taken in August 2021.
- LetchworthVillage.info
Albert Sabin‘s early work with attenuated-live-virus polio vaccine was developed from attenuated polio virus that Sabin had received from Koprowski.
In addition to his work on the polio vaccine, Koprowski (along with Stanley Plotkin and Tadeusz Wiktor) did significant work on an improved vaccine against rabies. The group developed the HDCV rabies vaccine in the 1960s at the Wistar Institute. It was licensed for use in the United States in 1980.
- “Rabies Vaccine 2”, History of Vaccines, The College of Physicians of Philadelphia (2017). Accessed 24 May 2017.
- Plotkin, S.A., “In Memoriam: Hilary Koprowski, 1916–2013”, J. Virol., August 2013 vol. 87 no. 15, pp. 8270-8271. doi: 10.1128/JVI.01449-13. Accessed 24 May 2017.
Koprowski was president of Biotechnology Foundation Laboratories, Inc, and head of the Center for Neurovirology at Thomas Jefferson University. In 2006 he was awarded a record 50th grant from the National Institutes of Health. He served as a consultant to the World Health Organization and the Pan American Health Organization.
British journalist Edward Hooper publicized a hypothesis that Koprowski’s research into a polio vaccine in the Belgian Congo in the late 1950s might have caused AIDS. The OPV AIDS hypothesis has, however, been rejected within much of the medical community and is contradicted by at least one article in the journal Nature, which claims the HIV-1 group M virus originated in Africa 30 years before the OPV trials were conducted. The journal Science refuted Hooper’s claims, writing: “[I]t can be stated with almost complete certainty that the large polio vaccine trial… was not the origin of AIDS.” Is it me or is this the worst debunking ever? Most of the medical community will do whatever their masters tell them to do so that is absolutely meaningless. So they are left with one article explaining why this scientific wonder was stomping around the Congo but definitely not bringing back plague and pestilence nobody ever heard of before but which was definitely documented in that area decades earlier? Documented by whom? And how exactly did they do that? Weren’t they still figuring out where hemorrhoids came from back then? What in hell is going on here? Fortunately, I don’t have time to dive into that pile of crazy right now but I will guess the biggest problem with those particular conspiracy theories is that they aren’t nearly big enough.
- Worobey M, Santiago ML, Keele BF, Ndjango JB, Joy JB, Labama BL, Dhed’A BD, Rambaut A, Sharp PM, Shaw GM, Hahn BH (2004). “Origin of AIDS: contaminated polio vaccine theory refuted” (PDF file, direct download 203 KB). Nature. 428 (6985): 820. Bibcode:2004Natur.428..820W. doi:10.1038/428820a. PMID 15103367. S2CID 4418410.
Note 1,13: Korber, B.et al. Science 288, 1789–1796 (2000)
- Panel nixes Congo trials as AIDS source”. Science. 258 (5083): 738–39. 1992. doi:10.1126/science.258.5083.738-d. PMID 1439779.
Koprowski rejected the claim, based on his own analysis. In a separate court case, he won a regretful clarification, and a symbolic award of $1 in damages, in a defamation suit against Rolling Stone, which had published an article repeating similar false allegations. A concurrent defamation lawsuit that Koprowski brought against the Associated Press was settled several years later; the settlement’s terms were not publicly disclosed.
- “Origin of AIDS” update: Clarification Archived 2008-08-05 at the Wayback Machine, uow.edu.au, 9 December 1993, p. 39
- Brian Martin (2001) “The Politics of a Scientific Meeting: the Origin-of-AIDS Debate at the Royal Society” in Politics & the Life Sciences, pp. 119-130 online Archived 2008-08-02 at the Wayback Machine
- Hilary Koprowski (1992). “AIDS and the polio vaccine”. Science. 257 (5073): 1024, 1026–27. Bibcode:1992Sci…257.1024K. doi:10.1126/science.257.5073.1024. PMID 1509249. Archived from the original on 2008-08-21. Retrieved 2006-06-09.
Koprowski’s original reports from 1960 to 1961 detailing part of his vaccination campaign in the Belgian Congo are available online from the World Health Organization.
- LeBrun A, Cerf J, Gelfand HM, Courtois G, Plotkin SA, Koprowski H (1960) “Vaccination with the CHAT strain of type 1 attenuated poliomyelities virus in Leopoldville, Belgian Congo 1. Description of the city, its history of poliomyelitis, and the plan of the vaccination campaign”, Bull World Health Organ. 22:203-13 online Archived 2008-10-31 at the Wayback Machine
- Plotkin SA, LeBrun A, Koprowski H (1960) “Vaccination with the CHAT strain of type 1 attenuated poliomyelitis virus in Leopoldville. Belgian Congo 2. Studies of the safety and efficacy of vaccination”, Bull World Health Organ 22:215-34 online Archived 2012-02-06 at the Wayback Machine
- Plotkin SA, LeBrun A, Courtois G, Koprowski H (1961) “Vaccination with the CHAT strain of type 1 attenuated poliomyelitis virus in Leopoldville, Congo 3. Safety and efficacy during the first 21 months of study” Bull World Health Organ 24:785-92 online Archived 2012-02-06 at the Wayback Machine
Main article: Oral polio vaccine AIDS hypothesis
Koprowski received many honorary degrees, academic honors, and national decorations, including the Order of the Lion from the King of Belgium, the French Order of Merit for Research and Invention, a Fulbright Scholarship, and appointment as Alexander von Humboldt Professor at the Max Planck Institute for Biochemistry in Munich. In 1989 he received the San Marino Award for Medicine and the Nicolaus Copernicus Medal of the Polish Academy of Sciences in Warsaw.
Koprowski received numerous honors in Philadelphia, including the Philadelphia Cancer Research Award, the John Scott Award and, in May 1990, the most prestigious honor of his home city, the Philadelphia Award. He was a Fellow of the College of Physicians of Philadelphia, which in 1959 presented him with its Alvarenga Prize.
Koprowski was a member of the National Academy of Sciences, the American Academy of Arts and Sciences, the New York Academy of Sciences, and the Polish Institute of Arts and Sciences of America. He held foreign membership in the Yugoslav Academy of Sciences and Arts, the Polish Academy of Sciences, the Russian Academy of Medical Sciences, and the Finnish Society of Sciences and Letters.
- Directory [of] PIASA Members, p. 25.
On June 3, 1983, Koprowski received an honorary doctorate from the Faculty of Medicine at Uppsala University, Sweden.
On 22 March 1995, Koprowski was made a Commander of Finland‘s Order of the Lion by Finland’s president. On 13 March 1997 he received the Legion d’Honneur from the French government. On 29 September 1998 he was presented by Poland’s president with the Grand Cross of Poland’s Order of Merit.
On 25 February 2000 Koprowski was honored with a reception at Philadelphia‘s Thomas Jefferson University celebrating the 50th anniversary of the first administration of his oral polio vaccine. At the reception, he received commendations from the United States Senate, the Pennsylvania Senate, and Pennsylvania Governor Tom Ridge.
On 13 September 2004, Koprowski was presented with the Pioneer in NeuroVirology Award by the International Society for NeuroVirology at the 6th International Symposium on NeuroVirology held in Sardinia. On 1 May 2007, Koprowski was awarded the Albert Sabin Gold Medal by the Sabin Vaccine Institute in Baltimore, Maryland.
- Award Ceremony and speeches Archived 2008-10-02 at the Wayback Machine
In 2014 Drexel University established the Hilary Koprowski Prize in Neurovirology in honor of Dr. Koprowski’s contributions to the field of neurovirology. The prize is awarded annually in conjunction with the International Symposium on Molecular Medicine and Infectious Disease, which is sponsored by the Institute for Molecular Medicine and Infectious Disease (IMMID) within the Drexel University College of Medicine. During the Symposium, the prize recipient is asked to deliver an honorary lecture.
They sure do give a lot of awards and honors while everybody is sick and dying mostly on other side of the doors to their dementofests. Something is not at all right with all that. It probably goes without saying but I remain all but convinced there is not a thing these assholes are up to, then or now, worth a single life or even a hair on the head of the people.
See also
- Albert Sabin
- Discredited HIV/AIDS origins theories
- Jonas Salk
- List of Polish people
- Poles
- Polio vaccine
- Wistar Institute
So that brings us to Cyanamide and a few other things.
Cyanamide is an organic compound with the formula CN2H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol-deterrent drug. The molecule features a nitrile group attached to an amino group. Derivatives of this compound are also referred to as cyanamides, the most common being calcium cyanamide (CaCN2) which is also included in these notes.
- Thomas Güthner; Bernd Mertschenk (2006). Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_139.pub2.
Not to be confused with Cyanimide or Cyanogen
- Cyanimides are a group of chemical compounds sharing a common functional group with the general structure R1R2N-C≡N. This group can be regarded as a secondary amine with a cyano substituent. [Mindiola, Daniel J.; Tsai, Yi-Chou; Hara, Ryuichiro; Chen, Qinghao; Meyer, Karsten; Cummins, Christopher C. (2001). “Bimetallic μ-cyanoimide complexes prepared by NCN group transfer” (PDF). Chemical Communications: 125–126. doi:10.1039/b006517j.]
- Cyanogen was first synthesized in 1815 by Joseph Louis Gay-Lussac, who determined its empirical formula and named it. Gay-Lussac coined the word “cyanogène” from the Greek words κυανός (kyanos, blue) and γεννάω (gennao, I create), because cyanide was first isolated by the Swedish chemist Carl Wilhelm Scheele from the pigment “Prussian blue“.Gay-Lussac, J. L. (1815). “Recherches sur l’acide prussique”. Annales de Chimie (in French). 95: 136–231. Gay-Lussac names cyanogen on p. 163.] By the 1850s, cyanogen soap was used by photographers to remove silver stains from their hands.[Crookes, William, ed. (1859). Photographic News: A Weekly Record of the Process of the Photography. p. 11.] It attained importance with the growth of the fertilizer industry in the late 19th century and remains an important intermediate in the production of many fertilizers. It is also used as a stabilizer in the production of nitrocellulose. Cyanogen is commonly found in comets.[Cometary Poison Gas Geyser Heralds Surprises NASA, 2010-11-02.] In 1910 a spectroscopic analysis of Halley’s Comet found cyanogen in the comet’s tail, which led to public fear that the Earth would be poisoned as it passed through the tail. People in New York wore gas masks, and merchants sold quack-treatment “comet pills” claimed to neutralise poisoning.[Cometary Poison Gas Geyser Heralds Surprises NASA, 2010-11-02.] Because of the extremely diffuse nature of the tail, there was no effect when the planet passed through it.[Comet’s Poisonous Tail.][Halley’s Comet 100 years ago.] Like other cyanides, cyanogen is very toxic, as it readily undergoes reduction to cyanide, which poisons the cytochrome c oxidase complex, thus interrupting the mitochondrial electron transfer chain. Cyanogen gas is an irritant to the eyes and respiratory system. Inhalation can lead to headache, dizziness, rapid pulse, nausea, vomiting, loss of consciousness, convulsions, and death, depending on exposure.[Muir, G. D., ed. (1971). Hazards in the Chemical Laboratory. London: The Royal Institute of Chemistry.] For the Android distribution, see CyanogenMod.
Cyanamide – Tautomers and self-condensations
Containing both a nucleophilic and electrophilic site within the same molecule, cyanamide undergoes various reactions with itself. Cyanamide exists as two tautomers, one with the connectivity N≡C–NH2 and the other with the formula HN=C=NH (“carbodiimide” tautomer). The N≡C–NH2 form dominates, but in a few reactions (e.g. silylation) the diimide form appears to be important.
- Thomas Güthner; Bernd Mertschenk (2006). Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_139.pub2.
Cyanamide dimerizes to give 2-cyanoguanidine (dicyandiamide). This dimerization is hindered or reversed by acids and is inhibited by low temperatures. The cyclic trimer is called melamine.
- Thomas Güthner; Bernd Mertschenk (2006). Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_139.pub2.
Melamine
Like cyanamide, it contains 67% nitrogen by mass, and its derivatives have fire-retardant properties due to its release of nitrogen gas when burned or charred. Melamine can be combined with formaldehyde and other agents to produce melamine resins. Such resins are characteristically durable thermosetting plastic used in high pressure decorative laminates such as Formica, melamine dinnerware including cooking utensils, plates, plastic products, laminate flooring, and dry erase boards. Melamine foam is used as insulation, soundproofing material and in polymeric cleaning products, such as Magic Eraser.
- Nutrition, Center for Food Safety and Applied (December 19, 2022). “Melamine in Tableware Questions and Answers”. FDA.
Melamine-formaldehyde resin tableware was evaluated by the Taiwan Consumers’ Foundation to have 20,000 parts per billion of free melamine that could migrate out of the plastic into acidic foods if held at 160 °F for two hours, such as if food was kept heated in contact with it in an oven.
- Nutrition, Center for Food Safety and Applied (December 19, 2022). “Melamine in Tableware Questions and Answers”. FDA.
Melamine was once illegally added to baby formula in China, in order to increase the apparent protein content. Ingestion of melamine may lead to reproductive damage, or bladder or kidney stones, and bladder cancer. It is also an irritant when inhaled or in contact with the skin or eyes. The United Nations’ food standards body, the Codex Alimentarius Commission, has set the maximum amount of melamine allowed in powdered infant formula to 1 mg/kg and the amount of the chemical allowed in other foods and animal feed to 2.5 mg/kg. While not legally binding, the levels allow countries to ban importation of products with excessive levels of melamine.
- Scholl, Peter F.; Bergana, Marti Mamula; Yakes, Betsy Jean; Xie, Zhuohong; Zbylut, Steven; Downey, Gerard; Mossoba, Magdi; Jablonski, Joseph; Magaletta, Robert; Holroyd, Stephen E.; Buehler, Martin (July 19, 2017). “Effects of the Adulteration Technique on the Near-Infrared Detection of Melamine in Milk Powder”. Journal of Agricultural and Food Chemistry. 65 (28): 5799–5809. doi:10.1021/acs.jafc.7b02083. ISSN 0021-8561. PMID 28617599.
Etymology
The German word Melamin was coined by combining the words melam (a derivative of ammonium thiocyanate) and amine. Melamine is, therefore, unrelated etymologically to the root melas (μέλας, meaning ‘black’ in Greek), from which the words melanin, a pigment, and melatonin, a hormone, are formed.
- “Melamine”. The American Heritage Dictionary of the English Language (Fourth ed.). 2000. Archived from the original on December 1, 2008. Retrieved September 28, 2008.
- Bann, Bernard; Miller, Samuel A. (1958). “Melamines and derivatives of melamine”. Chemical Reviews. 58: 131–172. doi:10.1021/cr50019a004.
Uses
Plastics and building materials
In one large-scale application, melamine is combined with formaldehyde and other agents to produce melamine resins. Such resins are characteristically durable thermosetting plastic used in high-pressure decorative laminates such as Formica, melamine dinnerware, laminate flooring, and dry erase boards. Melamine cookware is not microwave-safe.
- Deim, H.; Matthias, G.; Wagner, R. A. (2012). “Amino Resins”. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_115.pub2. ISBN 978-3527306732.
- “Melamine in Tableware Questions and Answers”. United States: Food and Drug Administration. December 19, 2022. Retrieved December 21, 2022.
Foods and drinks should not be heated on melamine-based dinnerware in microwave ovens.
Melamine foam is used as insulation, soundproofing material and in polymeric cleaning products, such as Magic Eraser.
Melamine is one of the major components in Pigment Yellow 150, a colorant in inks and plastics.
Melamine also is used in the fabrication of melamine polysulfonate, used as a superplasticizer for making high-resistance concrete. Sulfonated melamine formaldehyde (SMF) is a polymer used as a cement admixture to reduce the water content in concrete while increasing the fluidity and the workability of the mix during handling and pouring. It results in concrete with a lower porosity and a higher mechanical strength, exhibiting an improved resistance to aggressive environments and a longer lifetime.
Fertilizers
Melamine was once envisioned as fertilizer for crops during the 1950s and 1960s because of its high nitrogen content (2/3). However, melamine is much more expensive to produce than other common nitrogen fertilizers, such as urea. The mineralization (degradation to ammonia) for melamine is slow, making this product both economically and scientifically impractical for use as a fertilizer.[citation needed]
- Hauck, R. D.; Stephenson, H. F. (1964). “Fertilizer Nitrogen Sources, Nitrification of Triazine Nitrogen”. Journal of Agricultural and Food Chemistry. 12 (2): 147–151. doi:10.1021/jf60132a014.
Fire-retardant additives
Melamine and its salts are used as fire-retardant additives in paints, plastics, and paper. A melamine fibre, Basofil, has low thermal conductivity, excellent flame resistance and is self-extinguishing; this makes it useful for flame-resistant protective clothing, either alone or as a blend with other fibres.
- Ashford, Robert D. (2011) Ashford’s Dictionary of Industrial Chemicals, 3rd ed. Wavelength. p. 5713. ISBN 9780952267430.
- “Melamine Fibres”. Polymer Properties Database.
Food additive
Melamine is sometimes illegally added to food products in order to increase the apparent protein content. Standard tests, such as the Kjeldahl and Dumas tests, estimate protein levels by measuring the nitrogen content, so they can be misled by the addition of nitrogen-rich compounds such as melamine. There are instruments available today which can differentiate melamine nitrogen from protein nitrogen.
- Moore, Jeffrey C.; Devries, Jonathan W.; Lipp, Markus; Griffiths, James C.; Abernethy, Darrell R. (2010). “Total Protein Methods and Their Potential Utility to Reduce the Risk of Food Protein Adulteration”. Comprehensive Reviews in Food Science and Food Safety. 9 (4): 330–357. doi:10.1111/j.1541-4337.2010.00114.x. PMID 33467839.
Medicine
Melamine derivatives of arsenical drugs are potentially important in the treatment of African trypanosomiasis.
- Barrett, Michael P.; Gilbert, Ian H. (2006). “Targeting of Toxic Compounds to the Trypanosome’s Interior”. Advances in Parasitology. Advances in Parasitology. Vol. 63. pp. 125–183. doi:10.1016/S0065-308X(06)63002-9. ISBN 9780120317639. PMID 17134653.
Non-protein nitrogen
Melamine use as non-protein nitrogen (NPN) for cattle was described in a 1958 patent. In 1978, however, a study concluded that melamine “may not be an acceptable non-protein N source for ruminants” because its hydrolysis in cattle is slower and less complete than other nitrogen sources such as cottonseed meal and urea.
- Colby, Robert W. and Mesler, Robert J. Jr. (1958) “Ruminant feed compositions”. U.S. Patent 2,819,968.
- Newton, G. L.; Utley, P. R. (1978). “Melamine as a Dietary Nitrogen Source for Ruminants”. Journal of Animal Science. 47 (6): 1338–1344. doi:10.2527/jas1978.4761338x.
Non-protein nitrogen (or NPN) is a term used in animal nutrition to refer collectively to components such as urea, biuret, and ammonia, which are not proteins but can be converted into proteins by microbes in the ruminant stomach. Due to their lower cost compared to plant and animal proteins, their inclusion in a diet can result in economic gain, but at too high levels cause a depression in growth and possible ammonia toxicity, as microbes convert NPN to ammonia first before using that to make protein.
NPN can also be used to artificially raise crude protein values, which are measured based on nitrogen content, as protein is about 16% nitrogen and the only major component of most food which contains nitrogen is protein. The source of NPN is typically a chemical feed additive, or sometimes chicken waste and cattle manure.
- http://jas.fass.org/cgi/reprint/43/1/201.pdf[permanent dead link]
- “Value of Dried Poultry Manure and Urea as Protein Supplements for Sheep Consuming Low Quality Tropical Hay — Gihad 42 (3): 706 — Journal of Animal Science”. Archived from the original on 2008-10-15. Retrieved 2008-01-15.
- “Nutritional and Economic Value of Animal Excreta — Smith and Wheeler 48 (1): 144 — Journal of Animal Science”. Archived from the original on 2008-09-06. Retrieved 2008-01-15.
- http://jas.fass.org/cgi/reprint/57/Supplement_2/221.pdf[permanent dead link]
Nonruminants such as cats, dogs and pigs (and humans) cannot utilize NPN. NPN are given to ruminants in the form of pelleted urea, ammonium phosphate and/or biuret. Sometimes slightly polymerized special urea-formaldehyde resin or a mixture of urea and formaldehyde (both are also known as formaldehyde-treated urea) is used in place of urea, because the former provides a better control on the nitrogen release. This practice is carried out in China and other countries, such as Finland, India and France.
- “Nonprotein Nitrogen Poisoning, Merck Veterinary Manual, 9th ed”. Merck Co. Retrieved 27 April 2007.
- “Untreated and formaldehyde-treated urea as nitrogen sources for young growing bulls”, J. Setala, L. Syrjala-Qvist, P. Aspila, Journal of the Scientific Agricultural Society of Finland vol.54, p53-62, 1982
- “Evaluation of slow release urea formaldehyde complexes (SRUFC’S) as partial substitutes of protein in crossbred calves”, V.K. Sharma, B.N. Gupta, Asian Journal of Dairy Research vol.4, p119-25, 1985
- “Influence of Niacin Supplementation on In Vivo Digestibility and Ruminal Digestion in Dairy Cows” M. Doreau and J. F. Ottou, Journal of Dairy Science, vol.79 (12) 1996
Cyanuric acid has also been used as NPN. For example, Archer Daniels Midland manufactures an NPN supplement for cattle, which contains biuret, triuret, cyanuric acid and urea. FDA permits a certain amount of cyanuric acid to be present in some additives used in animal feed and also drinking water.
- “Roughage Buster Plus: ingredients”. Archer Daniels Midland. Archived from the original on 12 February 2007. Retrieved 6 May 2007.
- “Feed-grade biuret”. Food and Drug Administration. 1 April 2006. Archived from the original on 29 September 2007. Retrieved 6 May 2007.
See also
- Nitrogen cycle
- Cyanamide
- Cyanuric acid
- Melamine
- 1,3,5-Triazine
- Triazines
- Chinese protein export contamination
Toxicity
The short-term lethal dose of melamine is on a par with common table salt, with an LD50 of more than 3 grams per kilogram of bodyweight. U.S. Food and Drug Administration (FDA) scientists explained that when melamine and cyanuric acid are absorbed into the bloodstream, they concentrate and interact in the urine-filled renal tubules, then crystallize and form large numbers of round, yellow crystals, which in turn block and damage the renal cells that line the tubes, causing the kidneys to malfunction and lead to kidney stones, kidney failure, and death. Signs of melamine toxicity can include irritability, blood in the urine, little to no urine, symptoms of kidney infection, or high blood pressure.
- “Melamine in milk by David Bradley”. Sciencebase. September 17, 2008. Retrieved September 27, 2008.
- Weise, Elizabeth (August 5, 2007). “Poison pet food woes seem to hit cats harder”. USA Today. Retrieved October 1, 2008.
- Nutrition, Center for Food Safety and Applied (December 19, 2022). “Melamine in Tableware Questions and Answers”. FDA.
- Nutrition, Center for Food Safety and Applied (December 19, 2022). “Melamine in Tableware Questions and Answers”. FDA.
The European Union set a standard for acceptable human consumption (tolerable daily intake or TDI) of melamine at 0.2 mg per kilogram of body mass (previously 0.5 mg/kg), Canada declared a limit of 0.35 mg/kg, and the US FDA’s limit was put at 0.063 mg/kg (previously 0.63 mg/kg). The World Health Organization‘s food safety director estimated that the amount of melamine a person could stand per day without incurring a bigger health risk, the TDI, was 0.2 mg per kilogram of body mass.
- Harrington, Rory (April 15, 2010). “EFSA cuts melamine TDI by 60 per cent”. FoodQualityNews.com. Retrieved April 16, 2010.
- Endreszl, Lara (December 10, 2008). “Safe Melamine Levels Named by World Health Organization”. Health News. Archived from the original on March 18, 2009.
Toxicity of melamine can be mediated by intestinal microbiota. In culture, Klebsiella terrigena, which rarely colonizes mammalian intestines, was shown to convert melamine to cyanuric acid directly. Rats colonized by K. terrigena showed greater melamine-induced kidney damage compared to those not colonized.
- Neergaard, Lauran (February 14, 2013). “Study Examines Why Most Survived China’s Melamine Scare”. Food Manufacturing News. Food Manufacturing. Retrieved February 22, 2013.
- Zheng, X.; et al. (2013). “Melamine-induced renal toxicity is mediated by the gut microbiota”. Science Translational Medicine. 5 (172): 172ra22. doi:10.1126/scitranslmed.3005114. PMID 23408055. S2CID 23408614.
Acute toxicity
Melamine is reported to have an oral median lethal dose (LD50) of 3248 mg/kg based on rat data. It is also an irritant when inhaled or in contact with the skin or eyes. The reported dermal LD50 is >1000 mg/kg for rabbits. A study by Soviet researchers in the 1980s suggested that melamine cyanurate, commonly used as a fire retardant, could be more toxic than either melamine or cyanuric acid alone. For rats and mice, the reported LD50 for melamine cyanurate was 4.1 g/kg (given inside the stomach) and 3.5 g/kg (via inhalation), compared to 6.0 and 4.3 g/kg for melamine and 7.7 and 3.4 g/kg for cyanuric acid respectively.
- “Flame Retardants Center: Melamine Compounds”. Specialchem4polymers.com. April 19, 2010. Archived from the original on September 22, 2008. Retrieved June 20, 2012.
- Babayan, A. A. and Aleksandryan, A. V. (1985). “Токсичные характеристики цианурата меламина, меламина и циануровой кислоты” [Toxicological characteristics of melamine cyanurate, melamine and cyanuric acid]. Zhurnal Eksperimental’noi I Klinicheskoi Meditsiny. 25: 345–249.
A toxicology study in animals conducted after recalls of contaminated pet food concluded that the combination of melamine and cyanuric acid in diet does lead to acute kidney injury in cats. A 2008 study produced similar experimental results in rats and characterized the melamine and cyanuric acid in contaminated pet food from the 2007 outbreak. A 2010 study from Lanzhou University attributed kidney failure in humans to uric acid stone accumulation after ingestion of melamine resulting in a rapid aggregation of metabolites such as cyanuric acid diamide (ammeline) and cyanuric acid.[29] A 2013 study demonstrated that melamine can be metabolized to cyanuric acid by gut bacteria. In particular, Klebsiella terrigena was determined to be a factor in melamine toxicity. In culture, K. terrigena was shown to convert melamine to cyanuric acid directly. Cyanuric acid was detected in the kidneys of rats administered melamine alone, and the concentration after Klebsiella colonization was increased.
- Puschner, B.; Poppenga, R. H.; Lowenstine, L. J.; Filigenzi, M. S.; Pesavento, P. A. (2007). “Assessment of Melamine and Cyanuric Acid Toxicity in Cats”. Journal of Veterinary Diagnostic Investigation. 19 (6): 616–24. doi:10.1177/104063870701900602. PMID 17998549.
- Dobson, R. L. M.; Motlagh, S.; Quijano, M.; Cambron, R. T.; Baker, T. R.; Pullen, A. M.; Regg, B. T.; Bigalow-Kern, A. S.; Vennard, T.; Fix, A.; Reimschuessel, R.; Overmann, G.; Shan, Y.; Daston, G. P. (2008). “Identification and Characterization of Toxicity of Contaminants in Pet Food Leading to an Outbreak of Renal Toxicity in Cats and Dogs”. Toxicological Sciences. 106 (1): 251–262. doi:10.1093/toxsci/kfn160. PMID 18689873.
- Zhang, Xiangbo; Bai, Jinliang; Ma, Pengcheng; Ma, Jianhua; Wan, Jianghou; Jiang, Bin (2010). “Melamine-induced infant urinary calculi: A report on 24 cases and a 1-year follow-up”. Urological Research. 38 (5): 391–5. doi:10.1007/s00240-010-0279-0. PMID 20517603. S2CID 23832448.
- Zheng, X.; et al. (2013). “Melamine-induced renal toxicity is mediated by the gut microbiota”. Science Translational Medicine. 5 (172): 172ra22. doi:10.1126/scitranslmed.3005114. PMID 23408055. S2CID 23408614.
Chronic toxicity
Ingestion of melamine may lead to reproductive damage, or bladder or kidney stones, which can lead to bladder cancer.
- “International Chemical Safety Card”. Cdc.gov. Archived from the original on June 5, 2012. Retrieved June 20, 2012.
- OSHA – Chemical sampling information
- WHO – Some Chemicals that Cause Tumors of the Kidney or Urinary Bladder in Rodents and Some Other Substances[page needed]
- Heck, Henry d’A.; Tyl, Rochelle W. (1985). “The induction of bladder stones by terephthalic acid, dimethyl terephthalate, and melamine (2,4,6-triamino-s-triazine) and its relevance to risk assessment”. Regulatory Toxicology and Pharmacology. 5 (3): 294–313. doi:10.1016/0273-2300(85)90044-3. PMID 3903881.
A study in 1953 reported that dogs fed 3% melamine for a year had the following changes in their urine: (1) reduced specific gravity, (2) increased output, (3) melamine crystalluria, and (4) protein and occult blood.
- Tusing, T.W. “Chronic Feeding – Dogs”, cited by “Summary of toxicity data – trichloromelamine” by California Environmental Protection Agency, last revised on February 4, 2002, URL Archived June 25, 2007, at the Wayback Machine Retrieved September 5, 2007
A survey commissioned by the American Association of Veterinary Laboratory Diagnosticians suggested that crystals formed in the kidneys when melamine combined with cyanuric acid, “don’t dissolve easily. They go away slowly, if at all, so there is the potential for chronic toxicity.”
- “Culprit in pet food deaths may be combination of contaminants”. Michigan State University. November 29, 2007. Retrieved December 7, 2007.
- “Proceedings of the American Association of Veterinarian Laboratory Diagnosticians 50th Annual Conference” (PDF). AAVLD. October 2007. Archived from the original (PDF) on September 20, 2018. Retrieved May 16, 2021.
- “Researchers examine contaminants in food, deaths of pets”. AVMA. November 2007. Archived from the original on November 22, 2007. Retrieved November 30, 2007.
Metabolism
Melamine is a metabolite of cyromazine, a pesticide. It has been reported that cyromazine can also be converted to melamine in plants.
- “Cyromazine” (PDF). European Medicines Agency. January 2001. Archived from the original (PDF) on October 10, 2008. Retrieved June 20, 2012.
- Lim, Lori O.; Scherer, Susan J.; Shuler, Kenneth D.; Toth, John P. (1990). “Disposition of cyromazine in plants under environmental conditions”. Journal of Agricultural and Food Chemistry. 38 (3): 860–864. doi:10.1021/jf00093a057.
- “Cyromazine” (PDF). Pesticide Residues in Food, 1992 Evaluations: Residues. Food & Agriculture Org. 1993. pp. 265–. ISBN 978-92-5-103341-8. Archived from the original (PDF) on October 21, 2012.
Treatment of urolithiasis
Fast diagnosis and treatment of acute obstructive urolithiasis may prevent the development of acute kidney failure. Urine alkalinization and stone liberalization have been reported to be the most effective treatments in humans.
- Zhang, Xiangbo; Bai, Jinliang; Ma, Pengcheng; Ma, Jianhua; Wan, Jianghou; Jiang, Bin (2010). “Melamine-induced infant urinary calculi: A report on 24 cases and a 1-year follow-up”. Urological Research. 38 (5): 391–5. doi:10.1007/s00240-010-0279-0. PMID 20517603. S2CID 23832448.
Regulation in food and feed
The United Nations’ food standards body, Codex Alimentarius Commission, has set the maximum amount of melamine allowed in powdered infant formula to 1 mg/kg and the amount of the chemical allowed in other foods and animal feed to 2.5 mg/kg. While not legally binding, the levels allow countries to ban importation of products with excessive levels of melamine.
- “International experts limit melamine levels in food”. World Health Organization. July 6, 2010. Archived from the original on July 7, 2010. Retrieved July 7, 2010.
Establishment of maximum levels will help governments differentiate between low levels of unavoidable melamine occurrence that do not cause health problems, and deliberate adulteration – thereby protecting public health without unnecessary impediments to international trade.
Synthesis and reactions
Melamine was first synthesized by the German chemist Justus von Liebig in 1834. In early production, first calcium cyanamide was converted into dicyandiamide, which was heated above its melting temperature to produce melamine. Today most industrial manufacturers use urea in the following reaction to produce melamine:
6 (NH2)2CO → C3H6N6 + 6 NH3 + 3 CO2
In the first step, urea decomposes into cyanic acid and ammonia:
(NH2)2CO → HNCO + NH3
Cyanic acid polymerizes to cyanuric acid, which condenses with the liberated ammonia forming melamine. The released water reacts with cyanic acid, which helps to drive the reaction:
6 HNCO + 3 NH3 → C3H6N6 + 3 CO2 + 3NH3
The above reaction can be carried out by either of two methods: catalyzed gas-phase production or high pressure liquid-phase production. In one method, molten urea is introduced onto a fluidized bed with catalyst for reaction. Hot ammonia gas is also present to fluidize the bed and inhibit deammonization. The effluent then is cooled. Ammonia and carbon dioxide in the off-gas are separated from the melamine-containing slurry. The slurry is further concentrated and crystallized to yield melamine. Major manufacturers and licensors such as Orascom Construction Industries, BASF, and Eurotecnica have developed some proprietary methods.
- Kirk-Othmer (1978). Kirk-Othmer encyclopedia of chemical technology. Vol. 7 (3rd ed.). pp. 303–304. ISBN 9780471485162.
The off-gas contains large amounts of ammonia. Therefore, melamine production is often integrated into urea production, which uses ammonia as feedstock.
Crystallization and washing of melamine generates a considerable amount of waste water, which may be concentrated into a solid (1.5–5% of the weight) for easier disposal. The solid may contain approximately 70% melamine, 23% oxytriazines (ammeline, ammelide, and cyanuric acid), 0.7% polycondensates (melem, melam, and melon). In the Eurotecnica process, however, there is no solid waste and the contaminants are decomposed to ammonia and carbon dioxide and sent as off gas to the upstream urea plant; accordingly, the waste water can be recycled to the melamine plant itself or used as clean cooling water make-up.
- Lahalih, Shawqui M.; Absi-Halabi, M. (1989). “Recovery of solids from melamine waste effluents and their conversion to useful products”. Industrial & Engineering Chemistry Research. 28 (4): 500–504. doi:10.1021/ie00088a020.
- “How a golden chemical became greeneer”, Nitrogen+Syngas, Issue 293, May–June 2008.
Melamine reacts with acid and related compounds to form melamine cyanurate and related crystal structures, which have been implicated as contaminants or biomarkers in Chinese protein adulterations.
Drug derivatives
Melamine is part of the core structure for a number of drugs including almitrine, altretamine, cyromazine, ethylhexyl triazone, iscotrizinol, meladrazine, melarsomine, melarsoprol, tretamine, trinitrotriazine, and others.
- Matsui, Kohji (1972). “Syntheses and Reactions of s-Triazine Derivatives”. Journal of Synthetic Organic Chemistry, Japan (in Japanese). 30 (1): 19–35. doi:10.5059/yukigoseikyokaishi.30.19. ISSN 0037-9980.
Production in mainland China
Between the late 1990s and early 2000s, both consumption and production of melamine grew considerably in mainland China. By early 2006, melamine production in mainland China is reported to be in “serious surplus”. Between 2002 and 2007, while the global melamine price remained stable, a steep increase in the price of urea (feedstock for melamine) has reduced the profitability of melamine manufacturing. Currently, China is the world’s largest exporter of melamine, while its domestic consumption still grows by 10% per year. However, reduced profit has already caused other joint melamine ventures to be postponed there.
- Ruilin, Wang (January 6, 2006). “Melamine capacity is serious surplus”. China Chemical Reporter. Retrieved April 21, 2007.
Surplus melamine has been an adulterant for feedstock and milk in mainland China for several years now because it can make diluted or poor quality material appear to be higher in protein content by elevating the total nitrogen content detected by some simple protein tests. Actions taken in 2008 by the Government of China have reduced the practice of adulteration, with the goal of eliminating it. As a result of the Chinese milk scandal, court trials began in December 2008 for six people involved in adding melamine in food products, ending in January 2009 with two of the convicts being sentenced to death and executed.
- “Tainted milk trial opens in China”. BBC. December 26, 2008. Retrieved January 7, 2009.
- “Chinese Milk Scam Duo Face Death”. BBC. January 22, 2009. Retrieved January 22, 2009.
Melamine poisoning by tainted food
Melamine has been involved in several food recalls after the discovery of severe kidney damage to children and pets poisoned by melamine-adulterated food.
2007 animal-feed recalls
Further information: 2007 pet food recalls and Chinese protein adulteration
In 2007, a pet food recall was initiated by Menu Foods and other pet food manufacturers who had found their products had been contaminated and caused serious illnesses or deaths in some of the animals that had eaten them. In March 2007, the US Food and Drug Administration reported finding white granular melamine in the pet food, in samples of white granular wheat gluten imported from a single source in China, Xuzhou Anying Biologic Technology as well as in crystalline form in the kidneys and in urine of affected animals. Further vegetable protein imported from China was later implicated.
- Dry food added to pet food recall list. CNN. March 30, 2007
- “Pet food recall”. AVMA. April 11, 2007. Archived from the original on April 15, 2007. Retrieved June 20, 2012.
- Press release by Natural Balance Pet Foods Archived May 11, 2012, at the Wayback Machine
- Melamine Pet Food Recall – Frequently Asked Questions. FDA.gov (Updated October 7, 2009)
- “FDA: Pet food recall”. Fda.gov. Retrieved June 20, 2012.
In April 2007, The New York Times reported that the addition of “melamine scrap” into fish and livestock feed to give the false appearance of a higher level of protein was an “open secret” in many parts of mainland China, reporting that this melamine scrap was being produced by at least one plant processing coal into melamine. Four days later, the New York Times reported that, despite the widely reported ban on melamine use in vegetable proteins in mainland China, at least some chemical manufacturers continued to report selling it for use in animal feed and in products for human consumption. Li Xiuping, a manager at Henan Xinxiang Huaxing Chemical in Henan Province, stated, “Our chemical products are mostly used for additives, not for animal feed. Melamine is mainly used in the chemical industry, but it can also be used in making cakes.” Shandong Mingshui Great Chemical Group, the company reported by the New York Times as producing melamine from coal, produces and sells both urea and melamine but does not list melamine resin as a product.
- Barboza, David; Barrionuevo, Alexei (April 30, 2007). “Filler in Animal Feed Is Open Secret in China”. The New York Times. Retrieved April 30, 2007.
- Barboza, David & Barrionuevo, Alexei (May 3, 2007). “China Makes Arrest in Pet Food Case”. The New York Times. Retrieved May 3, 2007.
- “Products”. Shandong Mingshui Great Chemical Group. Archived from the original on July 26, 2005. Retrieved April 30, 2007.
Another recall incident in 2007 involved melamine which had been purposely added as a binder to fish and livestock feed manufactured in the United States. This was traced to suppliers in Ohio and Colorado.
- Martin, Andrew (May 31, 2007). “Poison used in China is found in U.S.-made animal feed”. The New York Times. Retrieved June 1, 2007.
2008 Chinese outbreak
Further information: 2008 Chinese milk scandal
In September 2008, several companies, including Nestlé, were implicated in a scandal involving milk and infant formula which had been adulterated with melamine, leading to kidney stones and other kidney failure, especially among young children. By December 2008, nearly 300,000 people had become ill, with more than 50,000 infant hospitalizations and six infant deaths. In a study published in the New England Journal of Medicine, it was reported that melamine exposure increased the incidence of urinary tract stones by seven times in children. Melamine may have been added to fool government protein content tests after water was added to fraudulently dilute the milk. Because of melamine’s high nitrogen content (66% by mass versus approximately 10–12% for typical protein), it can cause the protein content of food to appear higher than the true value. Officials estimate that about 20% of the dairy companies tested in China sell products tainted with melamine. On January 22, 2009, three of those involved in the scandal (including one conditional sentence) were sentenced to death in a Chinese court.
- Scott McDonald (September 21, 2008). “Nearly 53,000 Chinese children sick from milk”. The Pantagraph. ISSN 2641-7634. Archived from the original on May 16, 2021. Retrieved May 16, 2021.
- Jane Macartney, China baby milk scandal spreads as sick toll rises to 13,000, The Times (September 22, 2008)
- “Toxicological and Health Aspects of Melamine and Cyanuric Acid” (PDF). WHO. 2009. Retrieved August 13, 2009.
- Guan, Na; Fan, Qingfeng; Ding, Jie; Zhao, Yiming; Lu, Jingqiao; Ai, Yi; Xu, Guobin; Zhu, Sainan; Yao, Chen; Jiang, Lina; Miao, Jing; Zhang, Han; Zhao, Dan; Liu, Xiaoyu; Yao, Yong (2009). “Melamine-Contaminated Powdered Formula and Urolithiasis in Young Children”. New England Journal of Medicine. 360 (11): 1067–74. doi:10.1056/NEJMoa0809550. PMID 19196669.
- “Fonterra says somebody sabotaged milk”. NZ Herald. September 15, 2008. Retrieved September 22, 2008.
- “Toxic milk toll rockets in China”. BBC News. September 15, 2008. Retrieved September 22, 2008.
- Tran, Tini (September 17, 2008). “6,200 Chinese babies ill, 3 die from tainted milk”. Yahoo! News. Archived from the original on September 21, 2008. Retrieved September 22, 2008.
In October 2008, “Select Fresh Brown Eggs” exported to Hong Kong from the Hanwei Group in Dalian in northeastern China were found to be contaminated with nearly twice the legal limit of melamine. York Chow, the health secretary of Hong Kong, said he thought animal feeds might be the source of the contamination and announced that the Hong Kong Centre for Food Safety would henceforward be testing all mainland Chinese pork, farmed fish, animal feed, chicken meat, eggs, and offal products for melamine.
- “Hong Kong widens China food tests”. BBC News. October 27, 2008. Retrieved October 27, 2008.
As of July 2010, Chinese authorities were still reporting some seizures of melamine-contaminated dairy product in some provinces, though it was unclear whether these new contaminations constituted wholly new adulterations or were the result of illegal reuse of material from the 2008 adulterations.
- “Melamine tainted milk re-emerges in northwest China plant”. Xinhua. July 9, 2010. Archived from the original on July 11, 2010. Retrieved July 9, 2010.
- Wines, Michael (July 9, 2010). “Tainted Dairy Products Seized in Western China”. New York Times. Retrieved July 9, 2010.
On characterization and treatment of urinary stones in affected infants, The New England Journal of Medicine printed an editorial in March 2009, along with reports on cases from Beijing, Hong Kong and Taipei.
- Langman, Craig B. (2009). “Melamine, Powdered Milk, and Nephrolithiasis in Chinese Infants”. The New England Journal of Medicine. 360 (11): 1139–41. doi:10.1056/NEJMe0900361. PMID 19196666.
Urinary calculi specimens were collected from 15 cases treated in Beijing and were analyzed as unknown objects for their components at Beijing Institute of Microchemistry using infrared spectroscopy, nuclear magnetic resonance, and high performance liquid chromatography. The result of the analysis showed that the calculus was composed of melamine and uric acid, and the molecular ratio of uric acid to melamine was around 2:1.
- Sun, N.; Shen, Y.; Sun, Q.; Li, X. R.; Jia, L. Q.; Zhang, G. J.; Zhang, W. P.; Chen, Z.; Fan, J. F.; Jiang, Y. P.; Feng, D. C.; Zhang, R. F.; Zhu, X. Y.; Xiao, H. Z. (2009). “Diagnosis and treatment of melamine-associated urinary calculus complicated with acute renal failure in infants and young children”. Chinese Medical Journal. 122 (3): 245–51. PMID 19236798.
In a 2009 study of 683 children diagnosed in Beijing in 2008 with nephrolithiasis and 6,498 children without nephrolithiasis aged < 3 years, investigators found that in children exposed to melamine levels < 0.2 mg/kg per day, the risk for nephrolithiasis was 1.7 times higher than in those without melamine exposure, suggesting that the risk of melamine-induced nephrolithiasis in young children starts at a lower intake level than the levels recommended by the World Health Organization.
- Li, Gang; Jiao, Shufang; Yin, Xiangjun; Deng, Ying; Pang, Xinghuo; Wang, Yan (2009). “The risk of melamine-induced nephrolithiasis in young children starts at a lower intake level than recommended by the WHO”. Pediatric Nephrology. 25 (1): 135–41. doi:10.1007/s00467-009-1298-3. PMID 19727838. S2CID 360958.
In a study published in 2010, researchers from Beijing University studying ultrasound images of infants who fell ill in the 2008 contamination found that while most children in a rural Chinese area recovered, 12 per cent still showed kidney abnormalities six months later. “The potential for long-term complications after exposure to melamine remains a serious concern,” the report said. “Our results suggest a need for further follow-up of affected children to evaluate the possible long-term impact on health, including renal function.” Another 2010 follow-up study from Lanzhou University attributed the uric acid stone accumulation after ingestion of melamine to a rapid aggradation of metabolites such as cyanuric acid diamide (ammeline) and cyanuric acid and reported that urine alkalinization and stone liberalization were the most effective treatments.
- Liu, J.-m.; Ren, A.; Yang, L.; Gao, J.; Pei, L.; Ye, R.; Qu, Q.; Zheng, X. (2010). “Urinary tract abnormalities in Chinese rural children who consumed melamine-contaminated dairy products: A population-based screening and follow-up study”. Canadian Medical Association Journal. 182 (5): 439–43. doi:10.1503/cmaj.091063. PMC 2842835. PMID 20176755.
- Zhang, Xiangbo; Bai, Jinliang; Ma, Pengcheng; Ma, Jianhua; Wan, Jianghou; Jiang, Bin (2010). “Melamine-induced infant urinary calculi: A report on 24 cases and a 1-year follow-up”. Urological Research. 38 (5): 391–5. doi:10.1007/s00240-010-0279-0. PMID 20517603. S2CID 23832448.
Until the 2007 pet food recalls, melamine had not routinely been monitored in food, except in the context of plastic safety or insecticide residue.
Following the deaths of children in China from powdered milk in 2008, the Joint Research Centre (JRC) of the European Commission in Belgium set up a website about methods to detect melamine. In May 2009, the JRC published the results of a study that benchmarked the ability of labs around the world to accurately measure melamine in food. The study concluded that the majority of labs can effectively detect melamine in food.
- “About melamine”. Irmm.jrc.ec.europa.eu. February 2, 2012. Archived from the original on February 12, 2012. Retrieved June 20, 2012.
- Breidbach, A., Bouten, K., Kroger, K., Ulberth, F. “Melamine Proficiency Test 2009”. ec.europa.eu Archived October 15, 2009, at the Wayback Machine
In October 2008, the U.S. Food and Drug Administration (FDA) issued new methods for the analysis of melamine and cyanuric acid in infant formulations in the Laboratory Information Bulletin No 4421. Similar recommendations have been issued by other authorities, like the Japanese Ministry of Health, Labor and Welfare, both based on liquid chromatography – mass spectrometry (LC/MS) detection after hydrophilic interaction liquid chromatography (HILIC) separation.
- U.S. FDA Laboratory Information Bulletin No 4421 – “US FDA/CFSAN – Determination of Melamine and Cyanuric Acid Residues in Infant Formula using LC-MS/MS – Lib. 4421”. Archived from the original on October 10, 2008. Retrieved October 16, 2008.
- Japanese Ministry of Health, Labor and Welfare. forth.go.jp
- Zwitterionic HILIC separation of melamine and cyanuric acid – “Strategies for Determination of Melamine by HILIC”. October 3, 2008. Archived from the original on October 6, 2008. Retrieved October 16, 2008.
The existing methods for melamine determination using a triple quadrupole liquid chromatography – mass spectrometry (LC/MS) after solid phase extraction (SPE) are often complex and time-consuming. However, electrospray ionization methods coupled with mass spectrometry allow a rapid and direct analysis of samples with complex matrices: the native liquid samples are directly ionized under ambient conditions in their original solution. In December 2008, two new fast and inexpensive methods for detecting melamine in liquids have been published.
- Hodge, James (December 12, 2008). “Dairy detection: monitoring melamine in milk”. Chemical Science. No. 2. Royal Chemical Society, RCS Publishing. Retrieved January 4, 2009.
Ultrasound-assisted extractive electrospray ionization mass spectrometry (EESI-MS) has been developed at ETH Zurich (Switzerland) by Zhu, Chingin et al., (2008) for a rapid detection of melamine in untreated food samples. Ultrasounds are used to nebulize the melamine-containing liquids into a fine spray. The spray is then ionised by extractive electrospray ionisation (EESI) and analysed using tandem mass spectrometry (MS/MS). An analysis requires 30 seconds per sample. The limit of detection of melamine is a few nanograms of melamine per gram of milk.
- Zhu, Liang; Gamez, Gerardo; Chen, Huanwen; Chingin, Konstantin; Zenobi, Renato (2009). “Rapid detection of melamine in untreated milk and wheat gluten by ultrasound-assisted extractive electrospray ionization mass spectrometry (EESI-MS)”. Chemical Communications (5): 559–61. doi:10.1039/b818541g. PMID 19283290.
- Abedini, R.; Jahed Khaniki, G.; Molaee Aghaee, E.; Sadighara, P.; Nazmara, S.; Akbari-Adergani, B.; Naderi, M. (June 2021). “Determination of melamine contamination in chocolates containing powdered milk by high-performance liquid chromatography (HPLC)”. Journal of Environmental Health Science & Engineering. 19 (1): 165–171. doi:10.1007/s40201-020-00590-w. PMC 8172743. PMID 34150227.
Huang et al. (2008) have also developed at Purdue University (US) a simpler instrumentation and a faster method by using a low-temperature plasma probe to ionize the samples. The major obstacles being solved, the ESI-MS technique allows now high-throughput analysis of melamine traces in complex mixtures.
- Huang, Guangming; Ouyang, Zheng; Cooks, R. Graham (2009). “High-throughput trace melamine analysis in complex mixtures”. Chemical Communications (5): 556–8. doi:10.1039/b818059h. PMID 19283289.
The Melaminometer was a hypothetical design for a synthetic biology circuit, to be used for detecting melamine and related chemical analogues such as cyanuric acid. The conceptual project is hosted at OpenWetWare as open source biology in collaboration with DIYbio and has been discussed in various newspapers in the context of homebrew biotechnology. As of October 2009, the design has not been verified.
- Melaminometer
- McKenna, Phil (January 7, 2009). “Rise of the garage genome hackers”. New Scientist. Retrieved February 17, 2009.
- Marcus, Wohlsen (December 26, 2008). “Amateurs are trying genetic engineering at home”. Copyright 2008 The Associated Press. Retrieved February 17, 2009.
Because melamine resin is often used in food packaging and tableware, melamine at ppm level (1 part per million) in food and beverage has been reported due to migration from melamine-containing resins. Small amounts of melamine have also been reported in foodstuff as a metabolite product of cyromazine, an insecticide used on animals and crops.
- Ishiwata H, Inoue T, Yamazaki T, Yoshihira K (1987). “Liquid chromatographic determination of melamine in beverages”. Journal of the Association of Official Analytical Chemists. 70 (3): 457–460. doi:10.1093/jaoac/70.3.457. PMID 3610957.
- Sancho, J.V.; Ibáñez, M.; Grimalt, S.; Pozo, Ó.J.; Hernández, F. (2005). “Residue determination of cyromazine and its metabolite melamine in chard samples by ion-pair liquid chromatography coupled to electrospray tandem mass spectrometry”. Analytica Chimica Acta. 530 (2): 237–243. doi:10.1016/j.aca.2004.09.038. INIST:16514561.
Cyromazine is a triazine insect growth regulator used as an insecticide. It is a cyclopropyl derivative of melamine. Cyromazine works by affecting the nervous system of the immature larval stages of certain insects. In veterinary medicine, cyromazine is used as an ectoparasiticide.[citation needed] The Food Safety and Inspection Service (FSIS) of the United States Department of Agriculture (USDA) provides a test method for analyzing cyromazine and melamine in animal tissues in its Chemistry Laboratory Guidebook which “contains test methods used by FSIS Laboratories to support the Agency’s inspection program, ensuring that meat, poultry, and egg products are safe, wholesome and accurately labeled.” In 1999, in a proposed rule published in the Federal Register regarding cyromazine residue, the United States Environmental Protection Agency (EPA) proposed “remov[ing] melamine, a metabolite of cyromazine from the tolerance expression since it is no longer considered a residue of concern.”
- Merck Index, 12th Edition, 2845.
- Pesticide Fact Sheet from Pesticide Management Education Program, Cornell University
- “CYROMAZINE AND MELAMINE” (PDF). USDA FSIS. July 1991. Archived from the original (PDF) on 2007-06-16. Retrieved 2007-04-27.
- “Chemistry Laboratory Guidebook”. USDA FSIS. Retrieved 2007-04-27.
- Environmental Protection Agency. Cyromazine; Pesticide Tolerance
The Food Safety and Inspection Service (FSIS) of the United States Department of Agriculture (USDA) provides a test method for analyzing cyromazine and melamine in animal tissues. In 2007, the FDA began using a high performance liquid chromatography test to determine the melamine, ammeline, ammelide, and cyanuric acid contamination in food. Another procedure is based on surface-enhanced Raman spectroscopy (SERS).
- “Cyromazine and Melamine” (PDF). USDA FSIS. July 1991. Archived from the original (PDF) on June 16, 2007. Retrieved April 27, 2007.
- “Chemistry Laboratory Guidebook”. USDA FSIS. Retrieved April 27, 2007.
- “HPLC Determination of Melamine, Ammeline, Ammelide, and Cyanuric Acid Contamination in Wheat Gluten and Rice Protein Concentrate”. U.S. Food and Drug Administration. April 25, 2007. Retrieved May 9, 2007.
- He, Lili; Liu, Yang; Lin, Mengshi; Awika, Joseph; Ledoux, David R.; Li, Hao; Mustapha, Azlin (2008). “A new approach to measure melamine, cyanuric acid, and melamine cyanurate using surface enhanced Raman spectroscopy coupled with gold nanosubstrates”. Sensing and Instrumentation for Food Quality and Safety. 2: 66–71. doi:10.1007/s11694-008-9038-0. S2CID 93425738.
- Lin, M.; He, L.; Awika, J.; Yang, L.; Ledoux, D.R.; Li, H.; Mustapha, A. (2008). “Detection of Melamine in Gluten, Chicken Feed, and Processed Foods Using Surface Enhanced Raman Spectroscopy and HPLC”. Journal of Food Science. 73 (8): T129-34. doi:10.1111/j.1750-3841.2008.00901.x. PMID 19019134.
Member states of the European Union are required under Commission Decision 2008/757/EC to ensure that all composite products containing at least 15% of milk product, originating from China, are systematically tested before import into the Community and that all such products which are shown to contain melamine in excess of 2.5 mg/kg are immediately destroyed.
Detection in biological specimens
The presence of melamine in urine specimens from children who consumed adulterated milk products has been determined by liquid chromatography-mass spectrometry.
- Baselt RC (2014). Disposition of toxic drugs and chemicals in man. Seal Beach, Ca.: Biomedical Publications. pp. 1213–1214. ISBN 978-0-9626523-9-4.
Melamine on metal surfaces
It is reported that melamine molecules adsorbed on gold or silver surface tend to arrange into honeycomb or closed-packed structures. Such a self-assembly occurs due to the inter-molecular hydrogen bond interaction. This ordering was further investigated using classical Monte Carlo and DFT methods.
- Silly, Fabien; Shaw, Adam Q.; Castell, Martin R.; Briggs, G. A. D.; Mura, Manuela; Martsinovich, Natalia; Kantorovich, Lev (2008). “Melamine Structures on the Au(111) Surface”. J. Phys. Chem. C. 112 (30): 11476–11480. doi:10.1021/jp8033769.
- Schmitz, Christoph H.; Ikonomov, Julian; Sokolowski, Moritz (2011). “Two commensurate hydrogen-bonded monolayer structures of melamine on Ag(111)”. Surface Science. 605 (1–2): 1–6. Bibcode:2011SurSc.605….1S. doi:10.1016/j.susc.2010.09.006.
- Šimėnas, M.; Tornau, E. E. (2014). “A model of melamine molecules ordering on metal surfaces”. J. Chem. Phys. 141 (5): 054701. Bibcode:2014JChPh.141e4701A. doi:10.1063/1.4891245. PMID 25106594.
- Mura, M.; Martsinovich, N.; Kantorovich, L. (2008). “Theoretical study of melamine superstructures and their interaction with the Au(111) surface”. Nanotechnology. 19 (46): 465704. Bibcode:2008Nanot..19T5704M. doi:10.1088/0957-4484/19/46/465704. PMID 21836259. S2CID 25890258.
See also
Cyanamide Production
Cyanamide is produced by hydrolysis of calcium cyanamide, which in turn is prepared from calcium carbide via the Frank-Caro process.
CaCN2 + H2O + CO2 → CaCO3 + H2NCN
The conversion is conducted on slurries.
- Kurzer, Frederick; Lawson, Alexander (1954). “Methylisourea Hydrochloride”. Organic Syntheses. 34: 67. doi:10.15227/orgsyn.034.0067.
Reactions and uses
Cyanamide can be regarded as a functional single carbon fragment which can react as an electrophile or nucleophile. The main reaction exhibited by cyanamide involves additions of compounds containing an acidic proton. Water, hydrogen sulfide, and hydrogen selenide react with cyanamide to give urea, thiourea, and selenourea, respectively:
H2NCN + H2E → H2NC(E)NH2 (E = O, S, Se)
In this way, cyanamide behaves as a dehydration agent and thus can induce condensation reactions. Alcohols, thiols, and amines react analogously to give alkylisoureas, isothioureas, and guanidines. The anti-ulcer drug cimetidine is generated using such reactivity. Related reactions exploit the bifunctionality of cyanamide to give heterocycles, and this latter reactivity is the basis of several pharmaceutical syntheses such as the aminopyrimidine imatinib, and agrichemicals Amitrol and hexazinone. The hair-loss treatment minoxidil and the anthelmintics albendazole, flubendazole, and mebendazole feature 2-aminoimidazole substructures derived from cyanamide.
- Thomas Güthner; Bernd Mertschenk (2006). Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_139.pub2.
Cyanamide is also used in the synthesis of other pharmaceutical drugs including tirapazamine, etravirine, revaprazan, and dasantafil.
The cyanamide anion has the character of a pseudo chalcogen, cyanamide can therefore be regarded as analogue to water or hydrogen sulfide.
A convenient method for the preparation of secondary amines which are not contaminated with primary or tertiary amines is the reaction of cyanamide with alkyl halides to N,N-dialkylcyanamides which can easily be hydrolyzed to dialkylamines and then decarboxylated. Cyanamide adds itself in the presence of N-bromosuccinimide to olefinic double bonds. The addition product is converted by bases to N-Cyanaziridine, cyclized in the presence of acids to imidazolines, which can be further reacted to vicinal diamines by alkaline cleavage.
- Jonczyk A, Ochal Z, Makosza M (1978). “Reactions of Organic Anions; LXXXV1. Catalytic Two-Phase Alkylation of Cyanamide”. Synthesis. 1978 (12): 882–883. doi:10.1055/s-1978-24922.
- Ponsold K, Ihn W (1970). “Die Addition von cyanamid und Halogen an Olefine ein neues Verfahren zur Darstellung von vic.-Halogencyanaminen und Aziridinen”. Tetrahedron Lett. 11 (13): 1125–1128. doi:10.1016/S0040-4039(01)97925-0. PMID 5439242.
- Kohn, Harold; Jung, Sang Hun (1983). “New stereoselective method for the preparation of vicinal diamines from olefins and cyanamide”. Journal of the American Chemical Society. 105 (12): 4106–4108. doi:10.1021/ja00350a068..
Cyanamide is also a versatile synthetic building block for heterocycles: it forms 2-aminobenzimidazole with 1,2-diaminobenzene and it forms with the readily available cyclic enamine 4-(1-cyclohexenyl)morpholine and with elemental sulfur a 2-aminothiazole in good yields.
- Weiss, Stefan; Michaud, Horst; Prietzel, Horst; Krommer, Helmut (1973). “A New, Simple Synthesis of 2-Aminobenzimidazole”. Angewandte Chemie International Edition in English. 12 (10): 841. doi:10.1002/anie.197308411..
- S. Hünig, E. Lücke, and W. Brenninger (1961). “1-Morpholino-1-Cyclohexene”. Organic Syntheses: 65. doi:10.15227/orgsyn.041.0065.
- Gewald, K.; Spies, H.; Mayer, R. (1970). “Zur Reaktion von Enaminen mit Schwefel und Cyanamid” [On the Reaction of Enamines with Sulfur and Cyanamide]. Journal für Praktische Chemie. 312 (5): 776–779. doi:10.1002/prac.19703120507..
Sodium dicyanamide is available in good yield and high purity from cyanamid and cyanogen chloride, which is suitable as an intermediate for the synthesis of active pharmaceutical ingredients. A guanidino group is introduced by reaction of cyanamide with sarcosine In the industrial synthesis of creatine:

- E. B. Vliet (1925). “Diallylcyanamide”. Organic Syntheses. 5: 45. doi:10.15227/orgsyn.005.0045.
- Verfahren zur Herstellung von Natrium-Dicyanamid, veröffentlicht am 10. August 2000, Anmelder: SKW Trostberg AG.
- “Sodium dicyanamide (Na-dicyanamide)”. lonza.com. Archived from the original on 2013-05-23. Retrieved 2019-07-01.
- Deutsche Offenlegungsschrift DE-OS 10 2006 016 227 A1, Offenlegungsdatum: 11. Oktober 2007, Anmelder: Degussa GmbH.
This synthesis route mostly avoids problematic impurities like chloroacetic acid, iminodiacetic acid, or dihydrotriazine that occur in other routes. The physiological precursor guanidinoacetic is obtained analogously by reacting cyanamide with glycine.
Methods to stabilize cyanamidefmel make it available on an industrial scale. Due to the strong affinity towards self-condensation in alkaline media (see above) solutions of cyanamide are stabilized by the addition of 0.5 wt% of monosodium phosphate as buffer. Solid cyanamide is produced by careful evaporation of the solvent and subsequent addition of a hydrolysis-labile ester of formic acid. The ester absorbs traces of moisture (suppression of urea formation), neutralizes alkalinity (ammonia) and continually releases small amounts of formic acid.
- Wehrstedt, Klaus-Dieter; Wildner, Werner; Güthner, Thomas; Holzrichter, Klaus; Mertschenk, Bernd; Ulrich, Armin (2009-10-30). “Safe transport of cyanamide”. Journal of Hazardous Materials. 170 (2–3): 829–835. doi:10.1016/j.jhazmat.2009.05.043. ISSN 0304-3894. PMID 19505756
Agricultural use
Cyanamide, under the trade name Dormex, is a common agricultural rest-breaking agent applied in spring to stimulate uniform opening of buds, early foliation and bloom. Cyanamide can effectively compensate for the moderate lack of chilling units accumulated in the previous autumn and save the harvest that would otherwise be lost. It is particularly effective for woody plants such as blueberries, grapes, apples, peaches and kiwifruit. Most recently the product was approved for use on almonds and pistachios in the USA. Overdosage, high concentration and error in timing of application can damage the buds (especially of peach trees). Growers may avoid damage by applying 30 days prior to bud break according to the label.
- Powell, A. (1999). “Action Program for Dormex Application on Peaches”. Auburn University. Archived from the original on 2018-06-20.
Dormancy and rest-breaking agents
In plant physiology, dormancy is a period of arrested plant growth. It is a survival strategy exhibited by many plant species, which enables them to survive in harsh conditions and climates where part of the year is unsuitable for growth, such as winter or dry seasons.
Many plant species that exhibit dormancy have a biological clock that tells them when to slow activity and to prepare soft tissues for a period of freezing temperatures or water shortage. On the other hand, dormancy can be triggered after a normal growing season by decreasing temperatures, shortened day length, and/or a reduction in rainfall.
Chemical treatment on dormant plants has been proven to be an effective method to break dormancy, particularly in woody plants such as grapes, berries, apples, peaches, and kiwis.
Specifically, hydrogen cyanamide stimulates cell division and growth in dormant plants, causing buds to break when the plant is on the edge of breaking dormancy.[citation needed]
Slight injury of cells may play a role in the mechanism of action. The injury is thought to result in increased permeability of cellular membranes.[citation needed]
The injury is associated with the inhibition of catalase, which in turn stimulates the pentose phosphate cycle. Hydrogen cyanamide interacts with the cytokinin metabolic cycle, which results in triggering a new growth cycle.[citation needed]
Catalase
- Catalase is a common enzyme found in nearly all living organisms exposed to oxygen (such as bacteria, plants, and animals) which catalyzes the decomposition of hydrogen peroxide to water and oxygen.[Chelikani P, Fita I, Loewen PC (January 2004). “Diversity of structures and properties among catalases”. Cellular and Molecular Life Sciences. 61 (2): 192–208. doi:10.1007/s00018-003-3206-5. hdl:10261/111097. PMID 14745498. S2CID 4411482.] It is a very important enzyme in protecting the cell from oxidative damage by reactive oxygen species (ROS). Catalase has one of the highest turnover numbers of all enzymes; one catalase molecule can convert millions of hydrogen peroxide molecules to water and oxygen each second.[Goodsell DS (2004-09-01). “Catalase”. Molecule of the Month. RCSB Protein Data Bank. Retrieved 2016-08-23.] Catalase is a tetramer of four polypeptide chains, each over 500 amino acids long.[Boon EM, Downs A, Marcey D. “Catalase: H2O2: H2O2 Oxidoreductase”. Catalase Structural Tutorial Text. Retrieved 2007-02-11.] It contains four iron-containing heme groups that allow the enzyme to react with hydrogen peroxide. The optimum pH for human catalase is approximately 7,[Maehly AC, Chance B (1954). “The assay of catalases and peroxidases”. Methods of Biochemical Analysis. Methods of Biochemical Analysis. Vol. 1. pp. 357–424. doi:10.1002/9780470110171.ch14. ISBN 978-0-470-11017-1. PMID 13193536.] and has a fairly broad maximum: the rate of reaction does not change appreciably between pH 6.8 and 7.5.[Aebi H (1984). Catalase in vitro. Methods in Enzymology. Vol. 105. pp. 121–6. doi:10.1016/S0076-6879(84)05016-3. ISBN 978-0-12-182005-3. PMID 6727660.] The pH optimum for other catalases varies between 4 and 11 depending on the species.[ “EC 1.11.1.6 – catalase”. BRENDA: The Comprehensive Enzyme Information System. Department of Bioinformatics and Biochemistry, Technische Universität Braunschweig. Retrieved 2009-05-26.] The optimum temperature also varies by species.[Toner K, Sojka G, Ellis R. “A Quantitative Enzyme Study; CATALASE”. bucknell.edu. Archived from the original on 2000-06-12. Retrieved 2007-02-11.]
- HISTORY Catalase was first noticed in 1818 by Louis Jacques Thénard, who discovered hydrogen peroxide (H2O2). Thénard suggested its breakdown was caused by an unknown substance. In 1900, Oscar Loew was the first to give it the name catalase, and found it in many plants and animals.[Loew O (May 1900). “A New Enzyme of General Occurrence in Organisms”. Science. 11 (279): 701–702. Bibcode:1900Sci….11..701L. doi:10.1126/science.11.279.701. JSTOR 1625707. PMID 17751716.] In 1937 catalase from beef liver was crystallized by James B. Sumner and Alexander Dounce[Sumner JB, Dounce AL (April 1937). “Crystalline Catalase”. Science. 85 (2206): 366–367. Bibcode:1937Sci….85..366S. doi:10.1126/science.85.2206.366. PMID 17776781.] and the molecular weight was measured in 1938.[Sumner JB, Gralén N (March 1938). “The Molecular Weight of Crystalline Catalase”. Science. 87 (2256): 284. Bibcode:1938Sci….87..284S. doi:10.1126/science.87.2256.284. PMID 17831682. S2CID 36931581.] The amino acid sequence of bovine catalase was determined in 1969,[Schroeder WA, Shelton JR, Shelton JB, Robberson B, Apell G (May 1969). “The amino acid sequence of bovine liver catalase: a preliminary report”. Archives of Biochemistry and Biophysics. 131 (2): 653–655. doi:10.1016/0003-9861(69)90441-X. PMID 4892021.] and the three-dimensional structure in 1981.[Murthy MR, Reid TJ, Sicignano A, Tanaka N, Rossmann MG (October 1981). “Structure of beef liver catalase”. Journal of Molecular Biology. 152 (2): 465–499. doi:10.1016/0022-2836(81)90254-0. PMID 7328661.]
- MOLECULAR MECHANISM While the complete mechanism of catalase is not currently known,[18] the reaction is believed to occur in two stages:
- H2O2 + Fe(III)-E → H2O + O=Fe(IV)-E(.+)
- H2O2 + O=Fe(IV)-E(.+) → H2O + Fe(III)-E + O2[ Boon EM, Downs A, Marcey D. “Proposed Mechanism of Catalase”. Catalase: H2O2: H2O2 Oxidoreductase: Catalase Structural Tutorial. Retrieved 2007-02-11.]
- Here Fe()-E represents the iron center of the heme group attached to the enzyme. Fe(IV)-E(.+) is a mesomeric form of Fe(V)-E, meaning the iron is not completely oxidized to +V, but receives some stabilising electron density from the heme ligand, which is then shown as a radical cation (.+). As hydrogen peroxide enters the active site, it does not interact with the amino acids Asn148 (asparagine at position 148) and His75, causing a proton (hydrogen ion) to transfer between the oxygen atoms. The free oxygen atom coordinates, freeing the newly formed water molecule and Fe(IV)=O. Fe(IV)=O reacts with a second hydrogen peroxide molecule to reform Fe(III)-E and produce water and oxygen. The reactivity of the iron center may be improved by the presence of the phenolate ligand of Tyr358 in the fifth coordination position, which can assist in the oxidation of the Fe(III) to Fe(IV). The efficiency of the reaction may also be improved by the interactions of His75 and Asn148 with reaction intermediates.[Boon EM, Downs A, Marcey D. “Proposed Mechanism of Catalase”. Catalase: H2O2: H2O2 Oxidoreductase: Catalase Structural Tutorial. Retrieved 2007-02-11.] The decomposition of hydrogen peroxide by catalase proceeds according to first-order kinetics, the rate being proportional to the hydrogen peroxide concentration.[Aebi H (1984). “Catalase in vitro”. Methods in Enzymology. 105: 121–126. doi:10.1016/S0076-6879(84)05016-3. PMID 6727660.]
- Catalase can also catalyze the oxidation, by hydrogen peroxide, of various metabolites and toxins, including formaldehyde, formic acid, phenols, acetaldehyde and alcohols. It does so according to the following reaction: H2O2 + H2R → 2H2O + R The exact mechanism of this reaction is not known.
- Any heavy metal ion (such as copper cations in copper(II) sulfate) can act as a noncompetitive inhibitor of catalase. However, “Copper deficiency can lead to a reduction in catalase activity in tissues, such as heart and liver.”[Hordyjewska A, Popiołek Ł, Kocot J (August 2014). “The many “faces” of copper in medicine and treatment”. Biometals. 27 (4): 611–621. doi:10.1007/s10534-014-9736-5. PMC 4113679. PMID 24748564.] Furthermore, the poison cyanide is a noncompetitive inhibitor[ Nonstationary Inhibition of Enzyme Action. The Cyanide Inhibition of Catalase] of catalase at high concentrations of hydrogen peroxide.[Ogura Y, Yamazaki I (August 1983). “Steady-state kinetics of the catalase reaction in the presence of cyanide”. Journal of Biochemistry. 94 (2): 403–408. doi:10.1093/oxfordjournals.jbchem.a134369. PMID 6630165.] Arsenate acts as an activator.[ Kertulis-Tartar GM, Rathinasabapathi B, Ma LQ (October 2009). “Characterization of glutathione reductase and catalase in the fronds of two Pteris ferns upon arsenic exposure”. Plant Physiology and Biochemistry. 47 (10): 960–965. doi:10.1016/j.plaphy.2009.05.009. PMID19574057] Three-dimensional protein structures of the peroxidated catalase intermediates are available at the Protein Data Bank.
- CELLULAR ROLE Hydrogen peroxide is a harmful byproduct of many normal metabolic processes; to prevent damage to cells and tissues, it must be quickly converted into other, less dangerous substances. To this end, catalase is frequently used by cells to rapidly catalyze the decomposition of hydrogen peroxide into less-reactive gaseous oxygen and water molecules.[Gaetani GF, Ferraris AM, Rolfo M, Mangerini R, Arena S, Kirkman HN (February 1996). “Predominant role of catalase in the disposal of hydrogen peroxide within human erythrocytes”. Blood. 87 (4): 1595–1599. doi:10.1182/blood.V87.4.1595.bloodjournal8741595. PMID 8608252]
- Mice genetically engineered to lack catalase are initially phenotypically normal.[Ho YS, Xiong Y, Ma W, Spector A, Ho DS (July 2004). “Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury”. The Journal of Biological Chemistry. 279 (31): 32804–32812. doi:10.1074/jbc.M404800200. PMID 15178682.] However, catalase deficiency in mice may increase the likelihood of developing obesity, fatty liver,[Heit C, Marshall S, Singh S, Yu X, Charkoftaki G, Zhao H, et al. (February 2017). “Catalase deletion promotes prediabetic phenotype in mice”. Free Radical Biology & Medicine. 103: 48–56. doi:10.1016/j.freeradbiomed.2016.12.011. PMC 5513671. PMID 27939935.] and type 2 diabetes.[Góth L, Nagy T (September 2012). “Acatalasemia and diabetes mellitus”. Archives of Biochemistry and Biophysics. 525 (2): 195–200. doi:10.1016/j.abb.2012.02.005. PMID 22365890.] Some humans have very low levels of catalase (acatalasia), yet show few ill effects.
- The increased oxidative stress that occurs with aging in mice is alleviated by over-expression of catalase.[Selvaratnam J, Robaire B (November 2016). “Overexpression of catalase in mice reduces age-related oxidative stress and maintains sperm production”. Experimental Gerontology. 84: 12–20. doi:10.1016/j.exger.2016.08.012. PMID 27575890. S2CID 2416413.] Over-expressing mice do not exhibit the age-associated loss of spermatozoa, testicular germ and Sertoli cells seen in wild-type mice. Oxidative stress in wild-type mice ordinarily induces oxidative DNA damage (measured as 8-oxodG) in sperm with aging, but these damages are significantly reduced in aged catalase over-expressing mice.[Selvaratnam J, Robaire B (November 2016). “Overexpression of catalase in mice reduces age-related oxidative stress and maintains sperm production”. Experimental Gerontology. 84: 12–20. doi:10.1016/j.exger.2016.08.012. PMID 27575890. S2CID 2416413.] Furthermore, these over-expressing mice show no decrease in age-dependent number of pups per litter. Overexpression of catalase targeted to mitochondria extends the lifespan of mice.[Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, et al. (June 2005). “Extension of murine life span by overexpression of catalase targeted to mitochondria”. Science. 308 (5730): 1909–1911. Bibcode:2005Sci…308.1909S. doi:10.1126/science.1106653. PMID 15879174. S2CID 38568666.]
- In eukaryotes, catalase is usually located in a cellular organelle called the peroxisome.[Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). “Peroxisomes”. Molecular Biology of the Cell (4th ed.). New York: Garland Science. ISBN 978-0-8153-3218-3.] Peroxisomes in plant cells are involved in photorespiration (the use of oxygen and production of carbon dioxide) and symbiotic nitrogen fixation (the breaking apart of diatomic nitrogen (N2) to reactive nitrogen atoms). Hydrogen peroxide is used as a potent antimicrobial agent when cells are infected with a pathogen. Catalase-positive pathogens, such as Mycobacterium tuberculosis, Legionella pneumophila, and Campylobacter jejuni, make catalase to deactivate the peroxide radicals, thus allowing them to survive unharmed within the host.[Srinivasa Rao PS, Yamada Y, Leung KY (September 2003). “A major catalase (KatB) that is required for resistance to H2O2 and phagocyte-mediated killing in Edwardsiella tarda”. Microbiology. 149 (Pt 9): 2635–2644. doi:10.1099/mic.0.26478-0. PMID 12949187.]
- Like alcohol dehydrogenase, catalase converts ethanol to acetaldehyde, but it is unlikely that this reaction is physiologically significant.[Lieber CS (January 1997). “Ethanol metabolism, cirrhosis and alcoholism”. Clinica Chimica Acta; International Journal of Clinical Chemistry. 257 (1): 59–84. doi:10.1016/S0009-8981(96)06434-0. PMID 9028626.]
- DISTRIBUTION AMONG ORGANISMS The large majority of known organisms use catalase in every organ, with particularly high concentrations occurring in the liver in mammals.[Ilyukha VA (2001). “Superoxide Dismutase and Catalase in the Organs of Mammals of Different Ecogenesis”. Journal of Evolutionary Biochemistry and Physiology. 37 (3): 241–245. doi:10.1023/A:1012663105999. S2CID 38916410.] Catalase is found primarily in peroxisomes and the cytosol of erythrocytes (and sometimes in mitochondria[Bai J, Cederbaum AI (2001). “Mitochondrial catalase and oxidative injury”. Biological Signals and Receptors. 10 (3–4): 189–199. doi:10.1159/000046887. PMID 11351128. S2CID 33795198.])
- Almost all aerobic microorganisms use catalase. It is also present in some anaerobic microorganisms, such as Methanosarcina barkeri.[Brioukhanov AL, Netrusov AI, Eggen RI (June 2006). “The catalase and superoxide dismutase genes are transcriptionally up-regulated upon oxidative stress in the strictly anaerobic archaeon Methanosarcina barkeri”. Microbiology. 152 (Pt 6): 1671–1677. doi:10.1099/mic.0.28542-0. PMID 16735730.] Catalase is also universal among plants and occurs in most fungi.[Hansberg W, Salas-Lizana R, Domínguez L (September 2012). “Fungal catalases: function, phylogenetic origin and structure”. Archives of Biochemistry and Biophysics. 525 (2): 170–180. doi:10.1016/j.abb.2012.05.014. PMID 22698962.]
- One unique use of catalase occurs in the bombardier beetle. This beetle has two sets of liquids that are stored separately in two paired glands. The larger of the pair, the storage chamber or reservoir, contains hydroquinones and hydrogen peroxide, while the smaller, the reaction chamber, contains catalases and peroxidases. To activate the noxious spray, the beetle mixes the contents of the two compartments, causing oxygen to be liberated from hydrogen peroxide. The oxygen oxidizes the hydroquinones and also acts as the propellant.[Eisner T, Aneshansley DJ (August 1999). “Spray aiming in the bombardier beetle: photographic evidence”. Proceedings of the National Academy of Sciences of the United States of America. 96 (17): 9705–9709. Bibcode:1999PNAS…96.9705E. doi:10.1073/pnas.96.17.9705. PMC 22274. PMID 10449758.] The oxidation reaction is very exothermic (ΔH = −202.8 kJ/mol) and rapidly heats the mixture to the boiling point.[Beheshti N, McIntosh AC (2006). “A biomimetic study of the explosive discharge of the bombardier beetle” (PDF). International Journal of Design & Nature. 1 (1): 1–9. Archived from the original (PDF) on 2011-07-26.]
- Long-lived queens of the termite Reticulitermes speratus have significantly lower oxidative damage to their DNA than non-reproductive individuals (workers and soldiers). Queens have more than two times higher catalase activity and seven times higher expression levels of the catalase gene RsCAT1 than workers.[Tasaki E, Kobayashi K, Matsuura K, Iuchi Y (2017). “An Efficient Antioxidant System in a Long-Lived Termite Queen”. PLOS ONE. 12 (1): e0167412. Bibcode:2017PLoSO..1267412T. doi:10.1371/journal.pone.0167412. PMC 5226355. PMID 28076409.] It appears that the efficient antioxidant capability of termite queens can partly explain how they attain longer life.
- Catalase enzymes from various species have vastly differing optimum temperatures. Poikilothermic animals typically have catalases with optimum temperatures in the range of 15-25 °C, while mammalian or avian catalases might have optimum temperatures above 35 °C,[Mitsuda H (1956-07-31). “Studies on Catalase” (PDF). Bulletin of the Institute for Chemical Research, Kyoto University. 34 (4): 165–192. Archived (PDF) from the original on 2022-10-09. Retrieved 27 September 2017.][Çetinus ŞA, Öztop HN (June 2003). “Immobilization of catalase into chemically crosslinked chitosan beads”. Enzyme and Microbial Technology. 32 (7): 889–894. doi:10.1016/S0141-0229(03)00065-6.] and catalases from plants vary depending on their growth habit.[Mitsuda H (1956-07-31). “Studies on Catalase” (PDF). Bulletin of the Institute for Chemical Research, Kyoto University. 34 (4): 165–192. Archived (PDF) from the original on 2022-10-09. Retrieved 27 September 2017.] In contrast, catalase isolated from the hyperthermophile archaeon Pyrobaculum calidifontis has a temperature optimum of 90 °C.[Çetinus ŞA, Öztop HN (June 2003). “Immobilization of catalase into chemically crosslinked chitosan beads”. Enzyme and Microbial Technology. 32 (7): 889–894. doi:10.1016/S0141-0229(03)00065-6.]
- CLINICAL SIGNIFICANCE AND APPLICATION Catalase is used in the food industry for removing hydrogen peroxide from milk prior to cheese production.[“Catalase”. Worthington Enzyme Manual. Worthington Biochemical Corporation. Retrieved 2009-03-01.] Another use is in food wrappers, where it prevents food from oxidizing.[Hengge A (1999-03-16). “Re: how is catalase used in industry?”. General Biology. MadSci Network. Retrieved 2009-03-01.] Catalase is also used in the textile industry, removing hydrogen peroxide from fabrics to make sure the material is peroxide-free.[“textile industry”. Case study 228. International Cleaner Production Information Clearinghouse. Archived from the original on 2008-11-04. Retrieved 2009-03-01.] A minor use is in contact lens hygiene – a few lens-cleaning products disinfect the lens using a hydrogen peroxide solution; a solution containing catalase is then used to decompose the hydrogen peroxide before the lens is used again.[US patent 5521091, Cook JN, Worsley JL, “Compositions and method for destroying hydrogen peroxide on contact lens”, issued 1996-05-28]
- BACTERIAL IDENTIFICATION (CATALASE TEST) The catalase test is one of the three main tests used by microbiologists to identify species of bacteria. If the bacteria possess catalase (i.e., are catalase-positive), bubbles of oxygen are observed when a small amount of bacterial isolate is added to hydrogen peroxide. The catalase test is done by placing a drop of hydrogen peroxide on a microscope slide. An applicator stick is touched to the colony, and the tip is then smeared onto the hydrogen peroxide drop. If the mixture produces bubbles or froth, the organism is said to be ‘catalase-positive’. Staphylococci[Rollins DM (2000-08-01). “Bacterial Pathogen List”. BSCI 424 Pathogenic Microbiology. University of Maryland. Retrieved 2009-03-01.] and Micrococci[Johnson M. “Catalase Production”. Biochemical Tests. Mesa Community College. Archived from the original on 2008-12-11. Retrieved 2009-03-01.] are catalase-positive. Other catalase-positive organisms include Listeria, Corynebacterium diphtheriae, Burkholderia cepacia, Nocardia, the family Enterobacteriaceae (Citrobacter, E. coli, Enterobacter, Klebsiella, Shigella, Yersinia, Proteus, Salmonella, Serratia), Pseudomonas, Mycobacterium tuberculosis, Aspergillus, Cryptococcus, and Rhodococcus equi. If not, the organism is ‘catalase-negative’. Streptococcus[Fox A. “Streptococcus pneumoniae and Staphylococci”. University of South Carolina. Retrieved 2009-03-01.] and Enterococcus spp. are catalase-negative. While the catalase test alone cannot identify a particular organism, it can aid identification when combined with other tests such as antibiotic resistance. The presence of catalase in bacterial cells depends on both the growth condition and the medium used to grow the cells.
- BACTERIAL VIRULENCE Neutrophils and other phagocytes use peroxide to kill bacteria. The enzyme NADPH oxidase generates superoxide within the phagosome, which is converted via hydrogen peroxide to other oxidising substances like hypochlorous acid which kill phagocytosed pathogens.[Winterbourn CC, Kettle AJ, Hampton MB (June 2016). “Reactive Oxygen Species and Neutrophil Function”. Annual Review of Biochemistry. 85 (1): 765–792. doi:10.1146/annurev-biochem-060815-014442. PMID 27050287.] In individuals with chronic granulomatous disease (CGD), phagocytic peroxide production is impaired due to a defective NADPH oxidase system. Normal cellular metabolism will still produce a small amount of peroxide and this peroxide can be used to produce hypochlorous acid to eradicate the bacterial infection. However, if individuals with CGD are infected with catalase-positive bacteria, the bacterial catalase can destroy the excess peroxide before it can be used to produce other oxidising substances. In these individuals the pathogen survives and becomes a chronic infection. This chronic infection is typically surrounded by macrophages in an attempt to isolate the infection. This wall of macrophages surrounding a pathogen is called a granuloma. Many bacteria are catalase positive, but some are better catalase-producers than others. Some catalase-positive bacteria and fungi include: nocardia, pseudomonas, listeria, aspergillus, candida, E. coli, staphylococcus, serratia, B. cepacia and H. pylori.[ Le T, Bhushan V, Sochat M, Kallianos K, Chavda Y, Zureick AH (2017-01-06). First aid for the USMLE step 1 2017: a student-to-student guide (27th ed.). New York. ISBN 978-1-259-83762-3. OCLC 986222844.]
- ACATALASIA Acatalasia is a condition caused by homozygous mutations in CAT, resulting in a lack of catalase. Symptoms are mild and include oral ulcers. A heterozygous CAT mutation results in lower, but still present catalase.[“OMIM Entry – # 614097 – ACATALASEMIA”. www.omim.org.]
- GRAY HAIR Low levels of catalase may play a role in the graying process of human hair. Hydrogen peroxide is naturally produced by the body and broken down by catalase. Hydrogen peroxide can accumulate in hair follicles and if catalase levels decline, this buildup can cause oxidative stress and graying.[“Gray hair cure? Scientists find root cause of discoloration”. NBC News. 6 May 2013. Retrieved 2022-07-31.] These low levels of catalase are associated with old age. Hydrogen peroxide interferes with the production of melanin, the pigment that gives hair its color.[“Why Hair Turns Gray Is No Longer A Gray Area: Our Hair Bleaches Itself As We Grow Older”. Science News. ScienceDaily. 2009-02-24. Retrieved 2009-03-01.][Wood JM, Decker H, Hartmann H, Chavan B, Rokos H, Spencer JD, et al. (July 2009). “Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair”. FASEB Journal. 23 (7): 2065–2075. arXiv:0706.4406. doi:10.1096/fj.08-125435. PMID 19237503. S2CID 16069417.]
- INTERACTIONS Catalase has been shown to interact with the ABL2 and Abl genes.[Cao C, Leng Y, Kufe D (August 2003). “Catalase activity is regulated by c-Abl and Arg in the oxidative stress response”. The Journal of Biological Chemistry. 278 (32): 29667–29675. doi:10.1074/jbc.M301292200. PMID 12777400.] Infection with the murine leukemia virus causes catalase activity to decline in the lungs, heart and kidneys of mice. Conversely, dietary fish oil increased catalase activity in the heart, and kidneys of mice.[Xi S, Chen LH (2000). “Effects of dietary fish oil on tissue glutathione and antioxidant defense enzymes in mice with murine aids”. Nutrition Research. 20 (9): 1287–99. doi:10.1016/S0271-5317(00)00214-1.]
- METHODS FOR DETERMINING CATALAS ACTIVITY In 1870, Schoenn discovered a formation of yellow color from the interaction of hydrogen peroxide with molybdate; then, from the middle of the 20th century, this reaction began to be used for colorimetric determination of unreacted hydrogen peroxide in the catalase activity assay.[Razygraev AV (2021). “A method for measuring catalase activity in mosquitoes by using ammonium molybdate and reaction medium buffered with 3-(N-morpholino)propanesulfonic acid”. Parazitologiya. 55 (4): 318–336. doi:10.31857/S0031184721040049. ISSN 0031-1847. S2CID 237702049.] The reaction became widely used after publications by Korolyuk et al. (1988)[Koroliuk MA, Ivanova LI, Maĭorova IG, Tokarev VE (1988). “[A method of determining catalase activity]”. Laboratornoe Delo (1): 16–19. PMID 2451064.] and Goth (1991).[Góth L (February 1991). “A simple method for determination of serum catalase activity and revision of reference range”. Clinica Chimica Acta; International Journal of Clinical Chemistry. 196 (2–3): 143–151. doi:10.1016/0009-8981(91)90067-m. PMID 2029780.] Direct UV measurement of the decrease in the concentration of hydrogen peroxide is also widely used after the publications by Beers & Sizer[Beers RF, Sizer IW (March 1952). “A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase”. The Journal of Biological Chemistry. 195 (1): 133–140. doi:10.1016/S0021-9258(19)50881-X. PMID 14938361.] and Aebi.[Aebi H (1984-01-01). “Catalase in vitro”. Methods in Enzymology. Oxygen Radicals in Biological Systems. Academic Press. 105: 121–126. doi:10.1016/s0076-6879(84)05016-3. PMID 6727660.]
- See also
A 50% aqueous solution of cyanamide is also used as a biocide (disinfectant) particularly in pig farming, because it effectively kills salmonella and shigella and fights flies in all stages of development.
- “ALZOGUR®”. AlzChem (in German). Retrieved 2019-07-01.
Environmental aspects
Cyanamide degrades via hydrolysis to urea, an excellent fertilizer. Fungi, like Myrothecium verrucaria, accelerate this process utilizing the enzyme cyanamide hydratase.
- Stransky H, Amberger A (1973). “Isolierung und eigenschaften einer Cyanamid-hydratase (E.C.-Gruppe 4. 2.1.) aus Myrothecium verrucaria Alb. u. Schw” [Isolation and properties of a cyanamide hydratase (EC 4.2.1) from Myrothecium verrucaria]. Z. Pflanzenphysiol. 70: 74–87. doi:10.1016/S0044-328X(73)80049-2.
Cyanamide functional group
Cyanamide is the name for a functional group with the formula NCNRR’ where R and R’ can be a variety of groups. These compounds are called cyanamides. One example is naphthylcyanamide, C10H7N(H)CN Some cyanamides are prepared by alkylation of calcium cyanamide. Others, such as the naphthyl derivative, are produced indirectly.
- E. B. Vliet (1925). “Diallylcyanamide”. Organic Syntheses. 5: 45. doi:10.15227/orgsyn.005.0045.
- Homer W. J. Cressman (1947). “N-Methyl-1-Naphthylcyanamide”. Organic Syntheses. 27: 56. doi:10.15227/orgsyn.027.0056.
Cyanamide in space
Due to its high permanent dipole moment (i.e., 4.32 ± 0.08 D), cyanamide was detected in spectral emissions coming from the Sgr B2 molecular cloud (T < 100 K) through its microwave transitions as the first known interstellar molecule containing the NCN frame.
- Tyler, J.K.; Sheridan, J.; Costain, C.C. (August 1972). “The microwave spectra of cyanamide”. Journal of Molecular Spectroscopy. 43 (2): 248–261. doi:10.1016/0022-2852(72)90021-5.
- Turner, B. E.; Liszt, H. S.; Kaifu, N.; Kisliakov, A. G. (November 1975). “Microwave detection of interstellar cyanamide”. The Astrophysical Journal. 201: L149. Bibcode:1975ApJ…201L.149T. doi:10.1086/181963
Safety
It is used as an alcohol-deterrent drug in Canada, Europe, and Japan.
- Thomas Güthner; Bernd Mertschenk (2006). Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_139.pub2.
Cyanamide has a modest toxicity in humans. Workplace exposure to hydrogen cyanamide sprays or exposure in people living in the vicinity of spraying have been reported as causing respiratory irritation, contact dermatitis, headache, and gastrointestinal symptoms of nausea, vomiting, or diarrhea.
- Schep L, Temple W, Beasley M (January 2009). “The adverse effects of hydrogen cyanamide on human health: an evaluation of inquiries to the New Zealand National Poisons Centre”. Clinical Toxicology. Philadelphia, PA. 47 (1): 58–60. doi:10.1080/15563650802459254. PMID 18951270. S2CID 6961576.
Calcium cyanamide
Calcium cyanamide, also known as Calcium carbondiamide, Calcium cyan-2°-amide or Calcium cyanonitride is the inorganic compound with the formula CaCN2. It is the calcium salt of the cyanamide (CN2−2) anion. This chemical is used as fertilizer and is commercially known as nitrolime. It was first synthesized in 1898 by Adolph Frank and Nikodem Caro (Frank–Caro process).
- Auchmoody, L.R.; Wendel, G.W. (1973). “Effect of calcium cyanamide on growth and nutrition of plan fed yellow-poplar seedlings”. Res. Pap. Ne-265. Uppdr Darby, Pa: U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station. 11 P. U.S. Department of Agriculture, Forest Service. 265. Retrieved 2008-07-18.
- “History of Degussa: Rich harvest, healthy environment: Calcium cyanamide”. Archived from the original on 2006-10-19. Retrieved 2008-07-18.
The Frank–Caro process, also called cyanamide process, is the nitrogen fixation reaction of calcium carbide with nitrogen gas in a reactor vessel at about 1,000 °C. The reaction is exothermic and self-sustaining once the reaction temperature is reached. Originally the reaction took place in large steel cylinders with an electrical resistance element providing initial heat to start the reaction. Modern production uses rotating ovens. The synthesis produces a solid mixture of calcium cyanamide (CaCN2), also known as nitrolime, and carbon.
CaC2 + N2 → CaCN2 + C
The Frank–Caro process was the first commercial process that was used worldwide to fix atmospheric nitrogen. The product was used as fertilizer and commercially known as Lime-Nitrogen. Nitrolim or Kalkstickstoff in German.[“Muscle Shoals Alabama” (PDF). Historic American Engineering Record. Archived from the original (PDF) on 2014-02-22. Retrieved 2014-02-18.] The method was developed by the German chemists Adolph Frank and Nikodem Caro between 1895 and 1899. In its first decades, the world market for inorganic fertilizer was dominated by factories utilizing the cyanamide process.
The first full-scale factories were established in 1905 in Piano d´Orta (Italy) and Westeregeln (Germany). From 1908 the Frank–Caro process was used at North Western Cyanamide Company at Odda, Norway. With an annual production capacity of 12,000 ton from 1909, the factory at Odda was by far the largest in the world. At this time, first phase factories were established in Briançon (France), Martigny (Switzerland), Bromberg (Prussia/Poland) and Knapsack (Germany). The cyanamide factory at Odda ceased operation in 2002. It is still intact and is a Norwegian candidate to the UNESCO World Heritage List.[“Rjukan/Notodden and Odda/Tyssedal Industrial Heritage Sites, Hydro Electrical Powered Heavy Industries with associated Urban Settlements (Company Towns) and Transportation System”. UNESCO. Retrieved 2010-06-29.]
See also
- Odda process
- Birkeland–Eyde process
- Haber–Bosch process
- Linde–Frank–Caro process, a method to produce hydrogen from water gas
- Guide to the Papers of Adolf Frank (1834–1916) Archived 2011-07-25 at the Wayback Machine
- German patent nr. DE 88363 (1895)
In the 1920s the more energy-efficient Haber process gradually took over. In 1945 the production of calcium cyanamide reached a peak of an estimated 1.5 million tons a year.[ “Discovery of the Commercial Processes for Making Calcium Carbide and Acetylene”. National Historic Chemical Landmarks. American Chemical Society. Archived from the original on February 23, 2013. Retrieved June 25, 2012.]
In their search for a new process for producing cyanides for cyanide leaching of gold, Frank and Caro discovered the ability of alkaline earth carbides to absorb atmospheric nitrogen at high temperatures.
- Deutsches Reichspatent DRP 88363, “Verfahren zur Darstellung von Cyanverbindungen aus Carbiden”, Erfinder: A. Frank, N. Caro, erteilt am 31. März 1895.
Fritz Rothe, a colleague of Frank and Caro, succeeded in 1898 in overcoming problems with the use of calcium carbide and clarified that at around 1,100 °C not calcium cyanide but calcium cyanamide is formed in the reaction. In fact, the initial target product sodium cyanide can also be obtained from calcium cyanamide by melting it with sodium chloride in the presence of carbon:
CaCN2 + 2 NaCl + C → 2 NaCN + CaCl2
- H.H. Franck, W. Burg, Zeitschrift für Elektrochemie und angewandte physikalische Chemie, 40(10), 686-692 (Oktober 1934).
Frank and Caro developed this reaction for a large-scale, continuous production process. The process was particularly challenging due to the equipment requirements required by the high temperatures during the initial igniter step. This process requires meticulous temperature control since the melting point of calcium cyanamide is only about 120°C lower than the boiling point of sodium chloride.
In 1901, Ferdinand Eduard Polzeniusz patented a process that converts calcium carbide to calcium cyanamide in the presence of 10% calcium chloride at 700 °C. The advantage of lowering the reaction temperature by about 400 °C, however, must be weighed against the high amount of calcium chloride required and the discontinuous process control. Nevertheless, both processes (the Rothe–Frank–Caro process and the Polzeniusz-Krauss process) played a role in the first half of the 20th century. In the record year 1945, a total of approx. 1.5 million tonnes were produced worldwide using both processes. Frank and Caro also noted the formation of ammonia from calcium cyanamide.
- “Commercialization of Calcium Carbide and Acetylene – Landmark”. American Chemical Society. Retrieved 2019-01-31.
- Angewandte Chemie, Band 29, Ausgabe 16, Seite R97, 25. Februar 1916
CaCN2 + 3 H2O → 2 NH3 + CaCO3
Albert Frank recognized the fundamental importance of this reaction as a breakthrough in the provision of ammonia from atmospheric nitrogen and in 1901 recommended calcium cyanamide as a nitrogen fertilizer. Between 1908 and 1919, five calcium cyanamide plants with a total capacity of 500,000 tonnes per year were set up in Germany, and one in Switzerland.
- Eschenmooser, Walter (June 1997). “100 Years of Progress with LONZA”. CHIMIA. 51 (6): 259-269. doi:10.2533/chimia.1997.259. S2CID 100485418.
It was at the time the cheapest nitrogen fertilizer with additional efficacy against weeds and plant pests and had great advantages over conventional nitrogen fertilizers. However, the large-scale implementation of ammonia synthesis via the Haber process became a serious competitor to the very energy-intensive Frank Caro process. As urea (formed via the Haber–Bosch process) was significantly more nitrogen-rich (46% compared to ca. 20% nitrogen content) cheaper and faster acting, the role of calcium cyanamide was gradually reduced to a multifunctional nitrogen fertilizer in niche applications. Other reasons for its loss of popularity were its dirty-black color, dusty appearance and irritating properties, as well as its inhibition of an alcohol-degrading enzyme which causes temporary accumulation of acetaldehyde in the body leading to dizziness, nausea, and alcohol flush reaction when alcohol is consumed around the time of bodily exposure.
Production
Calcium cyanamide is prepared from calcium carbide. The carbide powder is heated at about 1000 °C in an electric furnace into which nitrogen is passed for several hours.
- Thomas Güthner; Bernd Mertschenk (2006). “Cyanamides”. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_139.pub2. ISBN 3527306730.
The product is cooled to ambient temperatures and any unreacted carbide is leached out cautiously with water.
CaC2 + N2 → CaCN2 + C (ΔHof = –69.0 kcal/mol at 25 °C)
It crystallizes in hexagonal crystal system with space group R3m and lattice constants a = 3.67 Å, c = 14.85 Å.
- F. Brezina, J. Mollin, R. Pastorek, Z. Sindelar. Chemicke tabulky anorganickych sloucenin (Chemical tables of inorganic compounds). SNTL, 1986.
- Vannerberg, N.G. “The crystal structure of calcium cyanamide” Acta Chemica Scandinavica (1-27,1973-42,1988) (1962) 16, p2263-p2266
Uses
The main use of calcium cyanamide is in agriculture as a fertilizer. In contact with water, it decomposes and liberates ammonia:
CaCN2 + 3 H2O → 2 NH3 + CaCO3
- Auchmoody, L.R.; Wendel, G.W. (1973). “Effect of calcium cyanamide on growth and nutrition of plan fed yellow-poplar seedlings”. Res. Pap. Ne-265. Uppdr Darby, Pa: U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station. 11 P. U.S. Department of Agriculture, Forest Service. 265. Retrieved 2008-07-18.
It was used to produce sodium cyanide by fusing with sodium carbonate:
CaCN2 + Na2CO3 + 2 C → 2 NaCN + CaO + 2 CO
Sodium cyanide is used in cyanide process in gold mining. It can also be used in the preparation of calcium cyanide and melamine.
Through hydrolysis in the presence of carbon dioxide, calcium cyanamide produces cyanamide:[clarification needed]
CaCN2 + H2O + CO2 → CaCO3 + H2NCN
The conversion is conducted in slurries. For this reason, most commercial calcium cyanamide is sold as an aqueous solution.
Thiourea can be produced by the reaction of hydrogen sulfide with calcium cyanamide in the presence of carbon dioxide.
- Mertschenk, Bernd; Beck, Ferdinand; Bauer, Wolfgang (2000). “Thiourea and Thiourea Derivatives”. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_803.pub3.
Calcium cyanamide is also used as a wire-fed alloy in steelmaking to introduce nitrogen into the steel.
Safety
The substance can cause alcohol intolerance, before or after the consumption of alcohol.
- Potential risks to human health and the environment from the use of calcium cyanamide as fertiliser, Scientific Committee on Health and Environmental Risks, 1,534 kB, March 2016, Retrieved 22 July 2017
American Cyanamid Company
American Cyanamid Company was a leading American conglomerate that became one of the nation’s top 100 manufacturing companies during the 1970s and 1980s, according to the Fortune 500 listings at the time.[citation needed] It started in fertilizer, but added many other lines of business. It merged with American Home Products in 1994. The combined company sold off most of its lines of business except pharmaceuticals, adopted the name of its remaining Wyeth division, and was bought by Pfizer in 2009, becoming defunct as a separate concern.
History
The company was founded by engineers Frank S. Washburn and Charles H. Baker in New York City in 1907, to capitalize on a German patent they had licensed for the manufacture of nitrogen products for fertilizer. The company’s name is derived from the chemical calcium cyanamide, the fertilizer they would manufacture. They soon set up headquarters in Nashville, investing a million dollars in several corporations underpinning the manufacturing operation to be set up in nearby Muscle Shoals, Alabama (sometimes called Mussel Shoals), 120 miles from Nashville, on the Tennessee River. These planned operations included an electric power generating company (Mussel Shoals Hydro-electric) a utility company to distribute the electricity that would power the chemical plant, and the Cyanamid manufacturing plant. Washburn was President and located in Nashville, while Baker was Vice President and remained in New York. Cynamide plants were also planned for Niagara Falls, Ontario and Georgia. By 1908 the company was incorporated in Maine. The Canadian plant was the first in operation in 1910, and was to be followed by the Alabama plant.
- “Nitrates From the Air”. The Florence Herald. 1907-05-31. p. 1. Retrieved 2022-03-28 – via Newspapers.com .
- “Cyanamid (part 1 of 2) – Will be Manufactured at Niagara Falls”. Nashville Banner. 1908-04-04. p. 1. Retrieved 2022-03-28.
- “Cyanamid (part 2 of 2)”. Nashville Banner. 1908-04-04. p. 12. Retrieved 2022-03-28.
- “Thos. A. Edison’s Dream of Atmospheric Fertilizer to Come True in the South”. Nashville Banner. 1907-06-18. p. 1. Retrieved 2022-03-28.
- “Fertilizer From Air”. The Commonwealth. 1907-07-04. p. 1. Retrieved 2022-03-28.
- Dexter, Ralph W. (1961-05-01). “Mussel shoals vs. muscle shoals”. Sterkiana. 4 (1). ISSN 0000-0000.
- “Stahlman Building Directory”. Nashville Banner. 1908-01-25. p. 3. Retrieved 2022-03-28.
- “Charles H. Baker”. Nashville Banner. 1908-03-04. p. 8. Retrieved 2022-03-28.
- “Offices To Be Located Here – American Cynamid Company Will Be in the Stahlman Building”. Nashville Banner. 1911-10-03. p. 9. Retrieved 2022-03-28.
- “General Offices Comes Back to Nashville”. Nashville Banner. 1911-12-02. p. 1. Retrieved 2022-03-28.
- “New Ontario Companies”. The Gazette. 1908-08-31. p. 7. Retrieved 2022-03-28.
- “President Benehan Cameron’s Annual Address”. The Farmer and Mechanic. 1909-11-09. p. 3. Retrieved 2022-03-28.
- “Nitrogen From Air”. The Los Angeles Times. 1910-01-16. p. 34. Retrieved 2022-03-28.
However, the development of United States manufacturing was suspended when they were denied the construction of a dam for the hydroelectric generation station. Instead, United States offices of the company imported product from its Canadian plant. The company abandoned its Nashville headquarters in 1915 and relocated them to New York City. At the same time, it was trying to raise political support, both grass-roots and via lobbying, to implement the Alabama power generation plan, and as it began to face competition for the American market.
- “Shall the State Surrender Another Right”. The Montgomery Advertiser. 1913-11-21. p. 4. Retrieved 2022-03-28.
- “Making the Air Contribute to the Support of Man – Obstacles”. The Tennessean. 1913-11-30. p. 40. Retrieved 2022-03-28.
- “Great Plant of the American Cyanamid Company”. The Montgomery Advertiser. 1915-07-11. p. 28. Retrieved 2022-03-28.
- “Remove Office of Cyanamid Company”. Nashville Banner. 1915-09-08. p. 9. Retrieved 2022-03-28.
- “Defeat of army appopriations bill section 82 (nitrate and hydroelectric plants at Mussel Shoals)”. Ottawa Evening Journal. 1916-03-29. p. 4. Retrieved 2022-03-29.
- “Nitric Acid Plant”. The Chronicle. 1916-01-10. p. 3. Retrieved 2022-03-29.
- “Advertisement by American Cyanamid (“Millions for Farmers…”)”. The Montgomery Advertiser. 1915-07-11. p. 28. Retrieved 2022-03-29.
- “Defeat of section 82 (federal appropriation for Muscle Shoals hydroelectric dam)”. Wisconsin State Journal. 1916-03-27. p. 8. Retrieved 2022-03-29.
In 1917, Cyanamid purchased the Ammo-Phosphate Corporation, which owned a fertilizer plant in Linden, New Jersey manufacturing ammonium phosphate.
- “Cyanamid Company Shows Large Gain”. The Gazette. 1917-08-14. p. 12. Retrieved 2022-03-29.
During World War I, the company shifted its nitrogen production from fertilizer to explosives. With offers of free use of patents and processes, along with personnel and equipment, it enticed the United States government to approve and pay for its original plans for the Alabama plant, with some modifications, to help with the war effort. A separate company, the Air Nitrates Corporation, was set up for this government contract to build and operate the plant for the duration of the war, with Cyanamid earning some fees and to later inherit the plant for the fertilizer business. This raised concerns of cronyism, but the critics were outnumbered by local supporters in Congress. However, when the war ended, the first stage of the plant had only just begun limited production. A few months later, the Justice Department began an investigation into the contract and possible graft.
- “Hydro Power Resuced to Explosive Makers”. National Post. 1918-01-05. p. 16. Retrieved 2022-04-01.
- “Spend Millions on Cyanamid Plant – Government Will Build a Plant East of Sheffield Near Dam No. 2”. The Progressive Age. 1917-12-14. p. 3. Retrieved 2022-04-01.
- “Mussel Shoals Developments (excerpt)”. The Commercial Appeal. 1918-03-31. p. 45. Retrieved 2022-04-01.
- “Congress May Inquire Into $100,000 [sic] Deal for Nitrogen”. Chicago Tribune. 1918-04-15. p. 4. Retrieved 2022-04-01.
- “Big Nitrate Plant Nearing Completion”. The Commercial Appeal. 1918-11-11. p. 6. Retrieved 2022-04-01.
- “More Developments In Muscle Shoals Probe – Contracting Company Capitalized at $10,000”. The Chattanooga News. 1919-02-10. p. 1. Retrieved 2022-04-01.
Washburn died October 9, 1922. At the time, the government still owned the Muscle Shoals plants. A year later, a number of interests were competing to buy or lease it, including Air Nitrates/American Cynamid, General Electric, and Henry Ford. However by 1926, the list of bidders was far different as the Senate debated the merits of Air Nitrates in a joint venture with Union Carbide, the local power companies (who were most interested in the generating station), and a New York financial consortium. In the end, after much lobbying and debate, none of the bids were accepted. The government elected to run the plants itself, balancing the regional power requirements against farming needs for inexpensive fertilizer.
- “Frank S. Washburn Dies; Built Third Ave. Railway”. The Standard Union. 1922-10-10. p. 3. Retrieved 2022-04-01.
- “F. S. Washburn Dead – Engineer was Pioneer In Extraction of Notrogen From Air”. Times Union. 1922-10-10. p. 3. Retrieved 2022-04-01.
- “New Muscle Shoals Offer Expected Soon”. The Falls City Journal. 1923-12-07. p. 1. Retrieved 2022-04-01.
- “Muscle Shoals Not Yet Nailed By Henry Ford”. Dayton Daily News. 1923-12-26. p. 10. Retrieved 2022-04-01.
- “SHoals Contest Being Narrowed”. The Birmingham News. 1926-04-15. p. 26. Retrieved 2022-04-01.
- “Two Firms Plan Joint Operation of Muscle Shoals”. The Atlanta Constitution. 1926-06-23. p. 1. Retrieved 2022-04-01.
- “Farm News”. Kansas State News. 1928-03-29. p. 4. Retrieved 2022-04-01.
The company grew to over 100,000 employees worldwide, and had over 200,000 shareholders by the mid-1970s. Its stock was traded on the New York Stock Exchange under the symbol ACY. It was repeatedly reorganized after the mid-1990s, merged with other firms, and saw brands and divisions sold or spun off. The bulk of the former company is now part of Pfizer, with smaller portions belonging to BASF, Procter & Gamble and other firms.
Product lines
Although originally a manufacturer of agricultural chemicals, the company broadened its product lines into many types of industrial chemicals and specialty chemicals. The company then diversified into synthetic fibers, pharmaceuticals, surgical products, plastics, and inorganic pigments before World War II; and later added, by acquisitions, cosmetic and toiletry products, perfumes, building products, home building, and several smaller product categories following World War II.
From 1931 to 1943 American Cyanamid produced the pesticide Zyklon B under license.
- Christianson, Scott (2010). The Last Gasp: The Rise and Fall of the American Gas Chamber. Berkeley: University of California Press. pp. 10, 92, 98. ISBN 978-0-520-25562-3.
Zyklon B (German: [translated Cyclone B) was the trade name of a cyanide-based pesticide invented in Germany in the early 1920s. It consisted of hydrogen cyanide (prussic acid), as well as a cautionary eye irritant and one of several adsorbents such as diatomaceous earth. The product is notorious for its use by Nazi Germany during the Holocaust to murder approximately 1.1 million people in gas chambers installed at Auschwitz-Birkenau, Majdanek, and other extermination camps. [A total of around 6 million Jews were murdered during the Holocaust. Evans, Richard J. (2008). The Third Reich At War. New York: Penguin Books. ISBN 978-0-14-311671-4.]
Hydrogen cyanide, a poisonous gas that interferes with cellular respiration, was first used as a pesticide in California in the 1880s. Research at Degesch of Germany led to the development of Zyklon (later known as Zyklon A), a pesticide that released hydrogen cyanide upon exposure to water and heat. It was banned after World War I, when Germany used a similar product as a chemical weapon. Degussa purchased Degesch in 1922. Their team of chemists, which included Walter Heerdt [de] and Bruno Tesch, devised a method of packaging hydrogen cyanide in sealed canisters along with a cautionary eye irritant and one of several adsorbents such as diatomaceous earth. The new product was also named Zyklon, but it became known as Zyklon B to distinguish it from the earlier version. Uses included delousing clothing and fumigating ships, warehouses, and trains.
The Nazis started using Zyklon B in extermination camps in early 1942 to murder prisoners during the Holocaust. Tesch, as well as his deputy executive, Karl Weinbacher, were executed in 1946 for knowingly selling the product to the SS for use on humans. Hydrogen cyanide is now rarely used as a pesticide but still has industrial applications. Firms in several countries continue to produce Zyklon B under alternative brand names, including Detia-Degesch, the successor to Degesch, who renamed the product Cyanosil in 1974.
Hydrogen cyanide is a poisonous gas that interferes with cellular respiration. Cyanide prevents the cell from producing adenosine triphosphate (ATP) by binding to one of the proteins involved in the electron transport chain. This protein, cytochrome c oxidase, contains several subunits and has ligands containing iron groups. The cyanide component of Zyklon B can bind at one of these iron groups, heme a3, forming a more stabilized compound through metal-to-ligand pi bonding. As a result of the formation of this new iron–cyanide complex, the electrons that would situate themselves on the heme a3 group can no longer do so. Instead, these electrons destabilize the compound; thus, the heme group no longer accepts them. Consequently, electron transport is halted, and cells can no longer produce the energy needed to synthesize ATP.[Nelson, David L.; Cox, Michael M. (2000). Lehninger Principles of Biochemistry. New York: Worth Publishers. ISBN 1-57259-153-6.] Death occurs in a human being weighing 68 kilograms (150 lb) within two minutes of inhaling 70 mg of hydrogen cyanide.[“Environmental and Health Effects”. International Cyanide Management Institute. Archived from the original on 30 November 2012. Retrieved 10 February 2017.][Hayes, Peter (2004). From Cooperation to Complicity: Degussa in the Third Reich. Cambridge; New York; Melbourne: Cambridge University Press. ISBN 0-521-78227-9.]
Hydrogen cyanide, discovered in the late 18th century, was used in the 1880s for the fumigation of citrus trees in California. Its use spread to other countries for the fumigation of silos, goods wagons, ships, and mills. Its light weight and rapid dispersal meant its application had to take place under tents or in enclosed areas.[Hayes, Peter (2004). From Cooperation to Complicity: Degussa in the Third Reich. Cambridge; New York; Melbourne: Cambridge University Press. ISBN 0-521-78227-9.] Research by Fritz Haber of the Kaiser Wilhelm Institute for Physical Chemistry and Electrochemistry led to the founding in 1919 of Deutsche Gesellschaft für Schädlingsbekämpfung mbH (Degesch), a state-controlled consortium formed to investigate military use of the chemical. Chemists at Degesch added a cautionary eye irritant to a less volatile cyanide compound which reacted with water in the presence of heat to become hydrogen cyanide. The new product was marketed as the pesticide Zyklon (cyclone). As a similar formula had been used as a weapon by the Germans during World War I, Zyklon was soon banned.[Hayes, Peter (2004). From Cooperation to Complicity: Degussa in the Third Reich. Cambridge; New York; Melbourne: Cambridge University Press. ISBN 0-521-78227-9.]
Deutsche Gold- und Silber-Scheideanstalt (German Gold and Silver Refinery; Degussa) became sole owners of Degesch in 1922. There, beginning in 1922, Walter Heerdt [de], Bruno Tesch, and others worked on packaging hydrogen cyanide in sealed canisters along with a cautionary eye irritant and adsorbent stabilizers such as diatomaceous earth. [Cautionary eye irritants used included chloropicrin and cyanogen chloride. Christianson, Scott (2010). The Last Gasp: The Rise and Fall of the American Gas Chamber. Berkeley: University of California Press. ISBN 978-0-520-25562-3.]
The new product was also labelled as Zyklon, but it became known as Zyklon B to distinguish it from the earlier version.[Hayes, Peter (2004). From Cooperation to Complicity: Degussa in the Third Reich. Cambridge; New York; Melbourne: Cambridge University Press. ISBN 0-521-78227-9.] Heerdt was named the inventor of Zyklon B in the Degesch patent application (number DE 438818) dated 20 June 1922. The Deutsches Patent- und Markenamt awarded the patent on 27 December 1926.[DE patent 438818, Heerdt, Dr Walter, “Verfahren zur Schaedlingsbekaempfung”, issued 27 December 1926, assigned to Deutsche Gesellschaft für Schädlingsbekämpfung mbH.] Beginning in the 1920s, Zyklon B was used at U.S. Customs facilities along the Mexican border to fumigate the clothing of border crossers.[Cockburn, Alexander (21 June 2007). “Zyklon B on the US Border”. The Nation. Retrieved 14 July 2021.][Burnett, John (January 28, 2006). “The Bath Riots: Indignity Along the Mexican Border”. NPR. Retrieved May 6, 2017.]
Corporate structure and marketing
In 1930, Degussa ceded 42.5 percent ownership of Degesch to IG Farben and 15 percent to Th. Goldschmidt AG, in exchange for the right to market pesticide products of those two companies through Degesch. Degussa retained managerial control.[Hayes, Peter (2004). From Cooperation to Complicity: Degussa in the Third Reich. Cambridge; New York; Melbourne: Cambridge University Press. ISBN 0-521-78227-9.]
While Degesch owned the rights to the brand name Zyklon and the patent on the packaging system, the chemical formula was owned by Degussa. Schlempe GmbH, which was 52 percent owned by Degussa, owned the rights to a process to extract hydrogen cyanide from waste products of sugar beet processing. This process was performed under license by two companies, Dessauer Werke and Kaliwerke Kolin, who also combined the resulting hydrogen cyanide with stabilizer from IG Farben and a cautionary agent from Schering AG to form the final product, which was packaged using equipment, labels, and canisters provided by Degesch.[Hayes, Peter (2004). From Cooperation to Complicity: Degussa in the Third Reich. Cambridge; New York; Melbourne: Cambridge University Press. ISBN 0-521-78227-9.] The finished goods were sent to Degesch, who forwarded the product to two companies that acted as distributors: Heerdt-Linger GmbH (Heli) of Frankfurt and Tesch & Stabenow (Testa) of Hamburg. Their territory was split along the Elbe river, with Heli handling clients to the west and south, and Testa those to the east.[Christianson, Scott (2010). The Last Gasp: The Rise and Fall of the American Gas Chamber. Berkeley: University of California Press. ISBN 978-0-520-25562-3.] Degesch owned 51 percent of the shares of Heli, and until 1942 owned 55 percent of Testa.[Hayes, Peter (2004). From Cooperation to Complicity: Degussa in the Third Reich. Cambridge; New York; Melbourne: Cambridge University Press. ISBN 0-521-78227-9.]
Prior to World War II Degesch derived most of its Zyklon B profits from overseas sales, particularly in the United States, where it was produced under license by Roessler & Hasslacher prior to 1931 and by American Cyanamid from 1931 to 1943.[Christianson, Scott (2010). The Last Gasp: The Rise and Fall of the American Gas Chamber. Berkeley: University of California Press. ISBN 978-0-520-25562-3.] From 1929, the United States Public Health Service used Zyklon B to fumigate freight trains and clothes of Mexican immigrants entering the United States.[Christianson, Scott (2010). The Last Gasp: The Rise and Fall of the American Gas Chamber. Berkeley: University of California Press. ISBN 978-0-520-25562-3.] Uses in Germany included delousing clothing (often using a portable sealed chamber invented by Degesch in the 1930s) and fumigating ships, warehouses, and trains. By 1943, sales of Zyklon B accounted for 65 percent of Degesch’s sales revenue and 70 percent of its gross profits.[Hayes, Peter (2004). From Cooperation to Complicity: Degussa in the Third Reich. Cambridge; New York; Melbourne: Cambridge University Press. ISBN 0-521-78227-9.]
Use in the Holocaust

In early 1942, the Nazis began using Zyklon B as the preferred killing tool in extermination camps during the Holocaust.[Longerich, Peter (2010). Holocaust: The Nazi Persecution and Murder of the Jews. Oxford; New York: Oxford University Press. ISBN 978-0-19-280436-5.] They used it to murder roughly 1.1 million people in gas chambers at Auschwitz-Birkenau, Majdanek, and elsewhere.[Hayes, Peter (2004). From Cooperation to Complicity: Degussa in the Third Reich. Cambridge; New York; Melbourne: Cambridge University Press. ISBN 0-521-78227-9.][“Auschwitz: Inside the Nazi State. Auschwitz 1940-1945. The Killing Evolution”. PBS. Retrieved 18 December 2019.] Most of the victims were Jews, and by far the majority of murders using this method took place at Auschwitz.[25][Hayes, Peter (2004). From Cooperation to Complicity: Degussa in the Third Reich. Cambridge; New York; Melbourne: Cambridge University Press. ISBN 0-521-78227-9.][Soviet officials initially stated that over 4 million people were killed using Zyklon B at Auschwitz, but this figure was proven to be greatly exaggerated. Steinbacher, Sybille (2005) [2004]. Auschwitz: A History. Munich: Verlag C. H. Beck. ISBN 0-06-082581-2.]
Distributor Heli supplied Zyklon B to Mauthausen, Dachau, and Buchenwald, and Testa supplied it to Auschwitz and Majdanek; camps also occasionally bought it directly from the manufacturers. Some 56 tonnes of the 729 tonnes sold in Germany in 1942–44 were sold to concentration camps, amounting to about 8 percent of domestic sales. Auschwitz received 23.8 tonnes, of which 6 tonnes were used for fumigation. The remainder was used in the gas chambers or lost to spoilage (the product had a stated shelf life of only three months). Testa conducted fumigations for the Wehrmacht and supplied them with Zyklon B. They also offered courses to the SS in the safe handling and use of the material for fumigation purposes. In April 1941, the German agriculture and interior ministries designated the SS as an authorized applier of the chemical, which meant they were able to use it without any further training or governmental oversight.[Hayes, Peter (2004). From Cooperation to Complicity: Degussa in the Third Reich. Cambridge; New York; Melbourne: Cambridge University Press. ISBN 0-521-78227-9.]

Rudolf Höss, commandant of Auschwitz, said that the use of Zyklon-B to murder prisoners came about on the initiative of one of his subordinates, SS-Hauptsturmführer (captain) Karl Fritzsch, who had used it to murder some Russian POWs in late August 1941 in the basement of Block 11 in the main camp. They repeated the experiment on more Russian POWs in September, with Höss watching.[Browning, Christopher R. (2004). The Origins of the Final Solution : The Evolution of Nazi Jewish Policy, September 1939 – March 1942. Comprehensive History of the Holocaust. Lincoln: University of Nebraska Press. ISBN 0-8032-1327-1.] Block 11 proved unsuitable, as the basement was difficult to air out afterwards and the crematorium (Crematorium I, which operated until July 1942) was some distance away.[Pressac, Jean-Claude; Pelt, Robert-Jan van (1994). “The Machinery of Mass Murder at Auschwitz”. In Gutman, Yisrael; Berenbaum, Michael (eds.). Anatomy of the Auschwitz Death Camp. Bloomington, Indiana: Indiana University Press. pp. 183–245. ISBN 0-253-32684-2.] The site of the murders was moved to Crematorium I, where more than 700 victims could be murdered at once. By the middle of 1942, the operation was moved to Auschwitz II–Birkenau, a nearby satellite camp that had been under construction since October 1941.[Piper, Franciszek (1994). “Gas Chambers and Crematoria”. In Gutman, Yisrael; Berenbaum, Michael (eds.). Anatomy of the Auschwitz Death Camp. Bloomington, Indiana: Indiana University Press. pp. 157–182. ISBN 0-253-32684-2.]
The first gas chamber at Auschwitz II–Birkenau was the “red house” (called Bunker 1 by SS staff), a brick cottage converted to a gassing facility by tearing out the inside and bricking up the windows. It was operational by March 1942. A second brick cottage, called the “white house” or Bunker 2, was converted some weeks later.[Rees, Laurence (2005). Auschwitz: A New History. New York: Public Affairs. ISBN 1-58648-303-X.][Piper, Franciszek (1994). “Gas Chambers and Crematoria”. In Gutman, Yisrael; Berenbaum, Michael (eds.). Anatomy of the Auschwitz Death Camp. Bloomington, Indiana: Indiana University Press. pp. 157–182. ISBN 0-253-32684-2.] According to Höss, Bunker 1 held 800 victims and Bunker 2 held 1,200 victims.[Piper, Franciszek (1994). “Gas Chambers and Crematoria”. In Gutman, Yisrael; Berenbaum, Michael (eds.). Anatomy of the Auschwitz Death Camp. Bloomington, Indiana: Indiana University Press. pp. 157–182. ISBN 0-253-32684-2.] These structures were in use for mass-murder until early 1943.[Steinbacher, Sybille (2005) [2004]. Auschwitz: A History. Munich: Verlag C. H. Beck. ISBN 0-06-082581-2.] At that point, the Nazis decided to greatly increase the gassing capacity of Birkenau. Crematorium II was originally designed as a mortuary with morgues in the basement and ground-level incinerators; they converted it into a killing factory by installing gas-tight doors, vents for the Zyklon B to be dropped into the chamber, and ventilation equipment to remove the gas afterwards.[Steinbacher, Sybille (2005) [2004]. Auschwitz: A History. Munich: Verlag C. H. Beck. ISBN 0-06-082581-2.][The gas chamber also had to be heated, as the Zyklon B pellets would not vaporize into hydrogen cyanide unless the temperature was 27 °C (81 °F) or above. Pressac, Jean-Claude; Pelt, Robert-Jan van (1994). “The Machinery of Mass Murder at Auschwitz”. In Gutman, Yisrael; Berenbaum, Michael (eds.). Anatomy of the Auschwitz Death Camp. Bloomington, Indiana: Indiana University Press. pp. 183–245. ISBN 0-253-32684-2.] Crematorium III was built using the same design. Crematoria IV and V, designed from the beginning as gassing centers, were also constructed that spring. By June 1943, all four crematoria were operational. Most of the victims were murdered using these four structures.[Rees, Laurence (2005). Auschwitz: A New History. New York: Public Affairs. ISBN 1-58648-303-X.]
The Nazis began shipping large numbers of Jews from all over Europe to Auschwitz in the middle of 1942. Those who were not selected for work crews were immediately gassed.[Pressac, Jean-Claude; Pelt, Robert-Jan van (1994). “The Machinery of Mass Murder at Auschwitz”. In Gutman, Yisrael; Berenbaum, Michael (eds.). Anatomy of the Auschwitz Death Camp. Bloomington, Indiana: Indiana University Press. pp. 183–245. ISBN 0-253-32684-2.] Those selected to die generally comprised about three-quarters of the total and included almost all children, women with small children, all the elderly, and all those who appeared on brief and superficial inspection by an SS doctor not to be completely fit.[Levy, Alan (2006) [1993]. Nazi Hunter: The Wiesenthal File (Revised 2002 ed.). London: Constable & Robinson. ISBN 978-1-84119-607-7.] The victims were told that they were to undergo delousing and a shower. They were stripped of their belongings and herded into the gas chamber.[Piper, Franciszek (1994). “Gas Chambers and Crematoria”. In Gutman, Yisrael; Berenbaum, Michael (eds.). Anatomy of the Auschwitz Death Camp. Bloomington, Indiana: Indiana University Press. pp. 157–182. ISBN 0-253-32684-2.]
A special SS bureau known as the Hygienic Institute delivered the Zyklon B to the crematoria by ambulance. The actual delivery of the gas to the victims was always handled by the SS, on the order of the supervising SS doctor. After the doors were shut, SS men dropped Zyklon B pellets through vents in the roof or holes in the side of the chamber. The victims were dead within 20 minutes. Johann Kremer, an SS doctor who oversaw gassings, testified that the “shouting and screaming of the victims could be heard through the opening and it was clear that they fought for their lives”.[Piper, Franciszek (1994). “Gas Chambers and Crematoria”. In Gutman, Yisrael; Berenbaum, Michael (eds.). Anatomy of the Auschwitz Death Camp. Bloomington, Indiana: Indiana University Press. pp. 157–182. ISBN 0-253-32684-2.]
Sonderkommandos (special work crews forced to work at the gas chambers) wearing gas masks then dragged the bodies from the chamber. The victims’ glasses, artificial limbs, jewelry, and hair were removed, and any dental work was extracted so the gold could be melted down. If the gas chamber was crowded, which they typically were, the corpses were found half-squatting, their skin discolored pink with red and green spots, with some foaming at the mouth or bleeding from their ears. The corpses were burned in the nearby incinerators, and the ashes were buried, thrown in the river, or used as fertilizer. With the Soviet Red Army approaching through Poland, the last mass gassing at Auschwitz took place on 30 October 1944.[Piper, Franciszek (1994). “Gas Chambers and Crematoria”. In Gutman, Yisrael; Berenbaum, Michael (eds.). Anatomy of the Auschwitz Death Camp. Bloomington, Indiana: Indiana University Press. pp. 157–182. ISBN 0-253-32684-2.] In November 1944, Reichsführer-SS Heinrich Himmler, head of the SS, ordered gassing operations to cease throughout the Reich.[Steinbacher, Sybille (2005) [2004]. Auschwitz: A History. Munich: Verlag C. H. Beck. ISBN 0-06-082581-2.]
Legacy

After World War II ended in 1945, Bruno Tesch and Karl Weinbacher of Tesch & Stabenow were tried in a British military court and executed for knowingly providing Zyklon B to the SS for use on humans.[Shirer, William L. (1960). The Rise and Fall of the Third Reich. New York: Simon & Schuster. ISBN 978-0-671-62420-0.] Gerhard Peters, who served as principal operating officer of Degesch and Heli and also held posts in the Nazi government, served two years and eight months in prison as an accessory before being released due to amendments to the penal code.[Hayes, Peter (2004). From Cooperation to Complicity: Degussa in the Third Reich. Cambridge; New York; Melbourne: Cambridge University Press. ISBN 0-521-78227-9.]
Use of hydrogen cyanide as a pesticide or cleaner has been banned or restricted in some countries.[United Nations Department of Economic and Social Affairs (2002). Consolidated List of Products Whose Consumption And/or Sale Have Been Banned, Withdrawn, Severely Restricted Or Not Approved by Governments: Chemicals. United Nations Publications. ISBN 978-92-1-130219-6.] Most hydrogen cyanide is used in industrial processes, made by companies in Germany, Japan, the Netherlands and the US.[Dzombak, David A.; Ghosh, Rajat S.; Wong-Chong, George M. (2005). Cyanide in Water and Soil: Chemistry, Risk, and Management. Boca Raton: CRC Press. ISBN 978-1-4200-3207-9.][United Nations Department of Economic and Social Affairs (2002). Consolidated List of Products Whose Consumption And/or Sale Have Been Banned, Withdrawn, Severely Restricted Or Not Approved by Governments: Chemicals. United Nations Publications. ISBN 978-92-1-130219-6.] Degesch resumed production of Zyklon B after the war. The product was sold as Cyanosil in Germany and Zyklon in other countries. It was still produced as of 2008.[“Bekanntmachung der geprüften und anerkannten Mittel und Verfahren zur Bekämpfung von tierischen Schädlingen nach §18 Infektionsschutzgesetz” [Notice of tested and approved means and procedures for combating animal pests according to §18, Infection Protection Act] (PDF). Bundesgesundheitsblatt: Bundesgesundheitsbl – Gesundheitsforsch – Gesundheitsschutz (in German). Bundesamtes für Verbraucherschutz und Lebensmittelsicherheit. 51. 20 June 2008. Archived from the original (PDF) on 23 February 2016. Retrieved 22 May 2018.] Degussa sold Degesch to Detia-Freyberg GmbH in 1986. The company is now called Detia-Degesch.[Hayes, Peter (2004). From Cooperation to Complicity: Degussa in the Third Reich. Cambridge; New York; Melbourne: Cambridge University Press. ISBN 0-521-78227-9.] Up until around 2015, a fumigation product similar to Zyklon B was in production by Lučební závody Draslovka of the Czech Republic, under the trade name Uragan D2. Uragan means “hurricane” or “cyclone” in Czech.[“Uragan D2” (in Czech). Lučební závody Draslovka a.s. Kolín. Archived from the original on 17 July 2015. Retrieved 7 July 2018.]
Cyanamid’s pharmaceutical division included “Lederle Laboratories”, maker of Piperacillin, an antibiotic drug used as a penicillin substitute; Centrum, a multivitamin supplement; Stresstabs vitamins; and Orimune, an oral polio vaccine.
- Chandler, Alfred Dupont (2005). Shaping the industrial century: the remarkable story of the evolution of the modern chemical and pharmaceutical industries. ISBN 978-0-674-01720-7.
Davis & Geck was the company’s medical device operation, organized under Lederle. Its Consumer Products division included “Shulton” products, primarily Old Spice cologne and after-shave lotion, Breck shampoo, and Pine-Sol household cleaner. A variety of fine fragrance products were made and sold by Shulton under license, including products under labels Nina Ricci, Pierre Cardin, Tabac, and others. “Melmac” was Cyanamid’s trademark for plastic kitchenware, although it was produced and marketed by other firms under license.
- Rappoport, Zvi (April 16, 2007). The chemistry of anilines. ISBN 978-0-470-87171-3.
- “Retro chic”. Archived from the original on April 22, 2005. Retrieved March 30, 2007.
Legal issues
Cyanamid was involved in the tetracycline litigation.
The discovery of tetracycline engendered an enormous amount of litigation. In late 1958, the U.S. government charged Pfizer and American Cyanamid with price fixing in connection with tetracycline. [“Government: Dissent on the Wonder Drugs,” Aug. 11, 1958, [1] and Pub Policy: Pricing Fixing.] That and other related litigation lasted until 1982. Often, the series of cases is referred to as the “antibiotics litigation.”
The focal point of the case was the two companies’ settlement of an interference proceeding before the PTO (the U.S. Patent and Trademark Office) over the priority of their respective applications for tetracycline. Under the settlement, American Cyanamid, which had acquired Heyden Chemicals‘s pending tetracycline application, conceded the priority of Pfizer’s application, withdrew its own application and exchanged cross licenses with Pfizer. According to the indictment of American Cyanamid, Bristol Myers, Pfizer, and Cyanamid knew that tetracycline represented a threat to the continuation of their dominant positions and unreasonably high profits. To keep that threat in check, the indictment alleges, Cyanamid bought out Heyden’s rights to the development and agreed to help Pfizer get the tetracycline patent. In return, charges the Justice Department, Pfizer licensed Cyanamid to produce the drug. Later, to avoid a court fight that might have nullified the patent, Pfizer and Cyanamid let Bristol-Myers in. [“Public Policy: Antitrust and & Antibiotics, August 25, 1961. [2]]
The Federal Trade Commission found that the cross-license, combined with the fact that Pfizer had withheld information that it knew or should have known was relevant to the patentability of tetracycline, constituted an attempt by Pfizer and American Cyanamid to share in an unlawful monopoly. [Cacciapaglia and Rockman, “Proposed Drug Industry Antitrust Act–Patents, Pricing, and the Public “ 30 Geo. Wash. L. Rev. 875, 894 (1961-1962); Kennedy, “Patent and Antitrust Policy–Acquisition of Patents by Fraud or by Unfair or Deceptive Acts or Practices,” 35 George Washington Law Review 512, 531 (1966-1967) For an early discussion, see Costello, “The Tetracycline Conspiracy: Structure, Conduct and Performance in the Drug Industry,” Antitrust L. & Economic Rev. 12 (1967-1968)] Ultimately, the government lost. [State of North Carolina v. Chas Pfizer & Co Inc. 537 F. 2d 67 (4th Cir. 1976).]
The government also sought to cancel the Pfizer patent on tetracycline, alleging fraud and the concealment of information that would have been relevant to the patent examiner. The government lost the final appeal of that case in 1982. [U.S. v. Pfizer,, 676 F.2d 51 (1982).]
See also
In its last years, the company was involved in numerous legal issues related to its earlier environmental pollution. During the 1970s, tens of millions of dollars were spent on effluent treatment – such as a $15-million tertiary water treatment plant in Bound Brook, New Jersey, which returned to the Raritan River water that was cleaner than the river itself, due to the river having been directly polluted by American Cyanamid, which had pumped toxic, undiluted liquid waste into the river for decades prior. Tens of millions more were spent in efforts to clean up large wastewater pools which had decades of accumulation of toxic, carcinogenic, and teratogenic chemicals. These are considered by the U.S. Environmental Protection Agency (EPA) to be among the most toxic chemical waste sites in the U.S. Cyanamid merged with American Home Products in 1994, and AHP changed its name to Wyeth which was then purchased by Pfizer in 2009. Responsibility for the clean-up of these sites remained with the site owner during these corporate transitions. Remediation began at Bound Brook in 2007 and Pfizer took over the site in 2009.
- American Cyanamid Bridgewater One-Page Summary Archived January 11, 2011, at the Wayback Machine
- Staff, Star-Ledger (7 September 2011). “Bridgewater superfund site still underwater following Hurricane Irene; tar balls feared”. nj.com.
- Impoundments 14 and 20 Closure Program[dead link]
- “Plans Being Shared on Cyanamid Remediation”. Bridgewater, NJ Patch. 6 December 2011.
The 575-acre Superfund site at Bound Brook-Bridgewater had a history of flooding. It was flooded in the 1930s and again in August 1971 during Hurricane Doria, at which time the plant sustained major damage to its facilities and equipment. In 2011, during Hurricane Irene the site once again flooded, but by this time all manufacturing had ended and all buildings had been torn down. However, impounds and wastesites remained with consequent leakage of benzene and numerous other chemicals into the Raritan River and adjacent land, apparently including residential sites. Subsequent testing showed no evident danger to humans, but the calamity intensified the extensive cleanup work already underway and the EPA announced another remediation plan for the site in September 2012.
- “American Cyanamid Superfund Site” (PDF). nj.gov.
- “09/28/2012: EPA Announces Remediation Plan for a Part of American Cyanamid Superfund Site in Bridgewater Township, N.J.” yosemite.epa.gov.
In the United Kingdom, the company was involved in a well-known legal case, American Cyanamid Co. (No.1) v Ethicon Ltd. (1975), which set the test for awarding an interim injunction in England and Wales and set down what became known to lawyers as the American Cyanamid principles. The American Cyanamid principles are also applied under public procurement law when the high court determines whether to lift the automatic suspension of the power to award a public contract when an application has been made to the court to challenge the lawfulness of a proposed contract award.
- American Cyanamid Co (No 1) v Ethicon Ltd [1975] UKHL 1, 5 February 1975, archived from the original on 20 May 2019, retrieved 21 December 2020
- Henderson Chambers, Group M UK Ltd. v Cabinet Office Archived 2016-04-11 at the Wayback Machine [2014] EWHC 3659 (TCC), published 17 March 2015, accessed 22 March 2016
Acquisition and breakup
The company merged with American Home Products (AHP) in 1994. At that time, the purchase price, $9.5 billion, made it the second-largest industrial acquisition in U.S. history to that point. American Home Products eventually changed its name to Wyeth Corporation (one of its subsidiaries), and in 2009 Wyeth merged with Pfizer, becoming a subsidiary of the world’s largest pharmaceutical company.
After the AHP acquisition, the Cyanamid conglomerate was disassembled over a period of years. The Pigments division was sold to National Lead Company. The Old Spice product line, and some others, were sold to Procter and Gamble. Formica Corporation was taken private in a management buyout, and later went through a series of ownership changes, and is owned by Fletcher Building, headquartered in New Zealand.
The $1.7 billion agricultural business was sold in 2000 to the German chemical giant BASF, raising BASF agricultural sales to $3.6 billion (1999 pro-forma), making it one of the top three agricultural companies in the world.
Most of the chemical businesses of American Cyanamid are operated by a spun-off successor company known as Cytec. Cytec was acquired by Solvay Group in December 2015 to form the Cytec Solvay Group based in Brussels, Belgium.
The American Cyanamid compound in Wayne, New Jersey later served as the headquarters of Toys “R” Us.
See also
General sources
- Ingham, John N (September 1983). Biographical dictionary of American business leaders. ISBN 978-0-313-23907-6.
- “American Chemical Industries”. Industrial & Engineering Chemistry. 22 (3): 301–302. 1930. doi:10.1021/ie50243a025.
- Kepos, Paula, ed. (December 17, 1993). International directory of company histories. Vol. 8. St. James Press. pp. 23–26. ISBN 155862323X.
- Richards, Bill (January 1, 1979). “Women Say They Had to Be Sterilized to Hold Jobs”.
- Rummel, Rudolph (1994). Death by Government. New Brunswick, NJ: Transaction. ISBN 978-1-56000-145-4.
- Snyder, Timothy (2010). Bloodlands: Europe Between Hitler and Stalin. New York: Basic Books. ISBN 978-0-465-00239-9.
- Roger Vaughan, Listen to the Music: The Life of Hilary Koprowski, Berlin, Springer, 2000; ISBN 0-387-98849-1
- David Oshinsky, Polio: An American Story, Oxford University Press, 2005; ISBN 0-19-515294-8
- 2007 Albert B. Sabin Gold Medal awarded to Hilary Koprowski (booklet/PDF file); accessed 21 April 2015.
- Directory [of] PIASA Members, 1999, New York City, Polish Institute of Arts and Sciences of America, 1999.
References
- Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- NIOSH Pocket Guide to Chemical Hazards. “#0091”. National Institute for Occupational Safety and Health (NIOSH).
- Auchmoody, L.R.; Wendel, G.W. (1973). “Effect of calcium cyanamide on growth and nutrition of plan fed yellow-poplar seedlings”. Res. Pap. Ne-265. Uppdr Darby, Pa: U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station. 11 P. U.S. Department of Agriculture, Forest Service. 265. Retrieved 2008-07-18.
- “History of Degussa: Rich harvest, healthy environment: Calcium cyanamide”. Archived from the original on 2006-10-19. Retrieved 2008-07-18.
- Deutsches Reichspatent DRP 88363, “Verfahren zur Darstellung von Cyanverbindungen aus Carbiden”, Erfinder: A. Frank, N. Caro, erteilt am 31. März 1895.
- H.H. Franck, W. Burg, Zeitschrift für Elektrochemie und angewandte physikalische Chemie, 40(10), 686-692 (Oktober 1934).
- “Commercialization of Calcium Carbide and Acetylene – Landmark”. American Chemical Society. Retrieved 2019-01-31.
- Angewandte Chemie, Band 29, Ausgabe 16, Seite R97, 25. Februar 1916
- Eschenmooser, Walter (June 1997). “100 Years of Progress with LONZA”. CHIMIA. 51 (6): 259-269. doi:10.2533/chimia.1997.259. S2CID 100485418.
- Thomas Güthner; Bernd Mertschenk (2006). “Cyanamides”. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_139.pub2. ISBN 3527306730.
- F. Brezina, J. Mollin, R. Pastorek, Z. Sindelar. Chemicke tabulky anorganickych sloucenin (Chemical tables of inorganic compounds). SNTL, 1986.
- Vannerberg, N.G. “The crystal structure of calcium cyanamide” Acta Chemica Scandinavica (1-27,1973-42,1988) (1962) 16, p2263-p2266
- Mertschenk, Bernd; Beck, Ferdinand; Bauer, Wolfgang (2000). “Thiourea and Thiourea Derivatives”. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_803.pub3.
- Potential risks to human health and the environment from the use of calcium cyanamide as fertiliser, Scientific Committee on Health and Environmental Risks, 1,534 kB, March 2016, Retrieved 22 July 2017
- “Muscle Shoals Alabama” (PDF). Historic American Engineering Record. Archived from the original (PDF) on 2014-02-22. Retrieved 2014-02-18.
- “Rjukan/Notodden and Odda/Tyssedal Industrial Heritage Sites, Hydro Electrical Powered Heavy Industries with associated Urban Settlements (Company Towns) and Transportation System”. UNESCO. Retrieved 2010-06-29.
- “Discovery of the Commercial Processes for Making Calcium Carbide and Acetylene”. National Historic Chemical Landmarks. American Chemical Society. Archived from the original on February 23, 2013. Retrieved June 25, 2012.
- “American Cyanamid History”. Funding Universe. Retrieved June 14, 2015.
- “Nitrates From the Air”. The Florence Herald. 1907-05-31. p. 1. Retrieved 2022-03-28 – via Newspapers.com .
- “Cyanamid (part 1 of 2) – Will be Manufactured at Niagara Falls”. Nashville Banner. 1908-04-04. p. 1. Retrieved 2022-03-28.
- “Cyanamid (part 2 of 2)”. Nashville Banner. 1908-04-04. p. 12. Retrieved 2022-03-28.
- “Thos. A. Edison’s Dream of Atmospheric Fertilizer to Come True in the South”. Nashville Banner. 1907-06-18. p. 1. Retrieved 2022-03-28.
- “Fertilizer From Air”. The Commonwealth. 1907-07-04. p. 1. Retrieved 2022-03-28.
- Dexter, Ralph W. (1961-05-01). “Mussel shoals vs. muscle shoals”. Sterkiana. 4 (1). ISSN 0000-0000.
- “Stahlman Building Directory”. Nashville Banner. 1908-01-25. p. 3. Retrieved 2022-03-28.
- “Charles H. Baker”. Nashville Banner. 1908-03-04. p. 8. Retrieved 2022-03-28.
- “Offices To Be Located Here – American Cynamid Company Will Be in the Stahlman Building”. Nashville Banner. 1911-10-03. p. 9. Retrieved 2022-03-28.
- “General Offices Comes Back to Nashville”. Nashville Banner. 1911-12-02. p. 1. Retrieved 2022-03-28.
- “New Ontario Companies”. The Gazette. 1908-08-31. p. 7. Retrieved 2022-03-28.
- “President Benehan Cameron’s Annual Address”. The Farmer and Mechanic. 1909-11-09. p. 3. Retrieved 2022-03-28.
- “Nitrogen From Air”. The Los Angeles Times. 1910-01-16. p. 34. Retrieved 2022-03-28.
- “Shall the State Surrender Another Right”. The Montgomery Advertiser. 1913-11-21. p. 4. Retrieved 2022-03-28.
- “Making the Air Contribute to the Support of Man – Obstacles”. The Tennessean. 1913-11-30. p. 40. Retrieved 2022-03-28.
- “Great Plant of the American Cyanamid Company”. The Montgomery Advertiser. 1915-07-11. p. 28. Retrieved 2022-03-28.
- “Remove Office of Cyanamid Company”. Nashville Banner. 1915-09-08. p. 9. Retrieved 2022-03-28.
- “Defeat of army appopriations bill section 82 (nitrate and hydroelectric plants at Mussel Shoals)”. Ottawa Evening Journal. 1916-03-29. p. 4. Retrieved 2022-03-29.
- “Nitric Acid Plant”. The Chronicle. 1916-01-10. p. 3. Retrieved 2022-03-29.
- “Advertisement by American Cyanamid (“Millions for Farmers…”)”. The Montgomery Advertiser. 1915-07-11. p. 28. Retrieved 2022-03-29.
- “Defeat of section 82 (federal appropriation for Muscle Shoals hydroelectric dam)”. Wisconsin State Journal. 1916-03-27. p. 8. Retrieved 2022-03-29.
- “Cyanamid Company Shows Large Gain”. The Gazette. 1917-08-14. p. 12. Retrieved 2022-03-29.
- “Hydro Power Resuced to Explosive Makers”. National Post. 1918-01-05. p. 16. Retrieved 2022-04-01.
- “Spend Millions on Cyanamid Plant – Government Will Build a Plant East of Sheffield Near Dam No. 2”. The Progressive Age. 1917-12-14. p. 3. Retrieved 2022-04-01.
- “Mussel Shoals Developments (excerpt)”. The Commercial Appeal. 1918-03-31. p. 45. Retrieved 2022-04-01.
- “Congress May Inquire Into $100,000 [sic] Deal for Nitrogen”. Chicago Tribune. 1918-04-15. p. 4. Retrieved 2022-04-01.
- “Big Nitrate Plant Nearing Completion”. The Commercial Appeal. 1918-11-11. p. 6. Retrieved 2022-04-01.
- “More Developments In Muscle Shoals Probe – Contracting Company Capitalized at $10,000”. The Chattanooga News. 1919-02-10. p. 1. Retrieved 2022-04-01.
- “Frank S. Washburn Dies; Built Third Ave. Railway”. The Standard Union. 1922-10-10. p. 3. Retrieved 2022-04-01.
- “F. S. Washburn Dead – Engineer was Pioneer In Extraction of Notrogen From Air”. Times Union. 1922-10-10. p. 3. Retrieved 2022-04-01.
- “New Muscle Shoals Offer Expected Soon”. The Falls City Journal. 1923-12-07. p. 1. Retrieved 2022-04-01.
- “Muscle Shoals Not Yet Nailed By Henry Ford”. Dayton Daily News. 1923-12-26. p. 10. Retrieved 2022-04-01.
- “SHoals Contest Being Narrowed”. The Birmingham News. 1926-04-15. p. 26. Retrieved 2022-04-01.
- “Two Firms Plan Joint Operation of Muscle Shoals”. The Atlanta Constitution. 1926-06-23. p. 1. Retrieved 2022-04-01.
- “Farm News”. Kansas State News. 1928-03-29. p. 4. Retrieved 2022-04-01.
- Christianson, Scott (2010). The Last Gasp: The Rise and Fall of the American Gas Chamber. Berkeley: University of California Press. pp. 10, 92, 98. ISBN 978-0-520-25562-3.
- Chandler, Alfred Dupont (2005). Shaping the industrial century: the remarkable story of the evolution of the modern chemical and pharmaceutical industries. ISBN 978-0-674-01720-7.
- Rappoport, Zvi (April 16, 2007). The chemistry of anilines. ISBN 978-0-470-87171-3.
- “Retro chic”. Archived from the original on April 22, 2005. Retrieved March 30, 2007.
- American Cyanamid Bridgewater One-Page Summary Archived January 11, 2011, at the Wayback Machine
- Staff, Star-Ledger (7 September 2011). “Bridgewater superfund site still underwater following Hurricane Irene; tar balls feared”. nj.com.
- Impoundments 14 and 20 Closure Program[dead link]
- “Plans Being Shared on Cyanamid Remediation”. Bridgewater, NJ Patch. 6 December 2011.
- “American Cyanamid Superfund Site” (PDF). nj.gov.
- “09/28/2012: EPA Announces Remediation Plan for a Part of American Cyanamid Superfund Site in Bridgewater Township, N.J.” yosemite.epa.gov.
- American Cyanamid Co (No 1) v Ethicon Ltd [1975] UKHL 1, 5 February 1975, archived from the original on 20 May 2019, retrieved 21 December 2020
- Henderson Chambers, Group M UK Ltd. v Cabinet Office Archived 2016-04-11 at the Wayback Machine [2014] EWHC 3659 (TCC), published 17 March 2015, accessed 22 March 2016
- “Auschwitz: Inside the Nazi State. Auschwitz 1940-1945. The Killing Evolution”. PBS. Retrieved 18 December 2019.
- Bailer-Gallanda, B. (1991). Amoklauf gegen die Wirklichkeit: NS-Verbrechen und “revisionistische” Geschichtsschreibung (in German). J. Bailer, F. Freund, T. Geisler, W. Lasek, N. Neugebauer, G. Spenn, W. Wegner. Wien: Bundesministerium fuer Unterricht und Kultur. ISBN 978-3-901142-07-9.
- “Bekanntmachung der geprüften und anerkannten Mittel und Verfahren zur Bekämpfung von tierischen Schädlingen nach §18 Infektionsschutzgesetz” [Notice of tested and approved means and procedures for combating animal pests according to §18, Infection Protection Act] (PDF). Bundesgesundheitsblatt: Bundesgesundheitsbl – Gesundheitsforsch – Gesundheitsschutz (in German). Bundesamtes für Verbraucherschutz und Lebensmittelsicherheit. 51. 20 June 2008. Archived from the original (PDF) on 23 February 2016. Retrieved 22 May 2018.
- Browning, Christopher R. (2004). The Origins of the Final Solution : The Evolution of Nazi Jewish Policy, September 1939 – March 1942. Comprehensive History of the Holocaust. Lincoln: University of Nebraska Press. ISBN 0-8032-1327-1.
- Burnett, John (January 28, 2006). “The Bath Riots: Indignity Along the Mexican Border”. NPR. Retrieved May 6, 2017.
- Christianson, Scott (2010). The Last Gasp: The Rise and Fall of the American Gas Chamber. Berkeley: University of California Press. ISBN 978-0-520-25562-3.
- Cockburn, Alexander (21 June 2007). “Zyklon B on the US Border”. The Nation. Retrieved 14 July 2021.
- “Cyclone B. La réaction de l’entreprise brestoise IPC”. Ouest-France (in French). 4 December 2013. Retrieved 6 August 2015.
- DE patent 438818, Heerdt, Dr Walter, “Verfahren zur Schaedlingsbekaempfung”, issued 27 December 1926, assigned to Deutsche Gesellschaft für Schädlingsbekämpfung mbH.
- Dzombak, David A.; Ghosh, Rajat S.; Wong-Chong, George M. (2005). Cyanide in Water and Soil: Chemistry, Risk, and Management. Boca Raton: CRC Press. ISBN 978-1-4200-3207-9.
- “Environmental and Health Effects”. International Cyanide Management Institute. Archived from the original on 30 November 2012. Retrieved 10 February 2017.
- Evans, Richard J. (2008). The Third Reich At War. New York: Penguin Books. ISBN 978-0-14-311671-4.
- “French Firm’s Cleaning Product Name Sounds Like Nazis’ Zyklon B”. The Jewish Press. 2 December 2013. Retrieved 6 August 2015.
- “Fury over Nazi gas sports shoe name”. BBC News. 29 August 2002. Retrieved 25 September 2014.
- Harmon, Brian; Stein, Mike (August 1994). “Prussian Blue: Why the Holocaust Deniers are Wrong”. The Nizkor Project. Archived from the original on 20 June 2014. Retrieved 25 September 2014.
- Hayes, Peter (2004). From Cooperation to Complicity: Degussa in the Third Reich. Cambridge; New York; Melbourne: Cambridge University Press. ISBN 0-521-78227-9.
- Katz, Leslie (August 6, 1999). “Does name of county fair ride throw Jews for a loop?”. J Weekly. San Francisco Jewish Community Publications. Retrieved 5 August 2015.
- Levy, Alan (2006) [1993]. Nazi Hunter: The Wiesenthal File (Revised 2002 ed.). London: Constable & Robinson. ISBN 978-1-84119-607-7.
- Longerich, Peter (2010). Holocaust: The Nazi Persecution and Murder of the Jews. Oxford; New York: Oxford University Press. ISBN 978-0-19-280436-5.
- Markiewicz, Jan; Gubala, Wojciech; Labedz, Jerzy (1994). “A Study of the Cyanide Compounds Content in the Walls of the Gas Chambers in the Former Auschwitz and Birkenau Concentration Camps”. Z Zagadnien Sqdowych. Institute for Forensic Research, Cracow (XXX): 17–27. Retrieved 25 September 2014.
- “Mr. Death: The Rise and Fall of Fred A. Leuchter, Jr. (film transcript)”. Fourth Floor Productions. 1999.
- Nelson, David L.; Cox, Michael M. (2000). Lehninger Principles of Biochemistry. New York: Worth Publishers. ISBN 1-57259-153-6.
- Piérot, Jean-Paul (5 December 2013). “Zyklon B, pardon. Cyclone B”. L’Humanité (in French). Retrieved 7 July 2018.
- Piper, Franciszek (1994). “Gas Chambers and Crematoria”. In Gutman, Yisrael; Berenbaum, Michael (eds.). Anatomy of the Auschwitz Death Camp. Bloomington, Indiana: Indiana University Press. pp. 157–182. ISBN 0-253-32684-2.
- Pressac, Jean-Claude; Pelt, Robert-Jan van (1994). “The Machinery of Mass Murder at Auschwitz”. In Gutman, Yisrael; Berenbaum, Michael (eds.). Anatomy of the Auschwitz Death Camp. Bloomington, Indiana: Indiana University Press. pp. 183–245. ISBN 0-253-32684-2.
- Rees, Laurence (2005). Auschwitz: A New History. New York: Public Affairs. ISBN 1-58648-303-X.
- Shirer, William L. (1960). The Rise and Fall of the Third Reich. New York: Simon & Schuster. ISBN 978-0-671-62420-0.
- “Siemens retreats over Nazi name”. BBC News. 5 September 2002. Retrieved 25 September 2014.
- Steinbacher, Sybille (2005) [2004]. Auschwitz: A History. Munich: Verlag C. H. Beck. ISBN 0-06-082581-2.
- United Nations Department of Economic and Social Affairs (2002). Consolidated List of Products Whose Consumption And/or Sale Have Been Banned, Withdrawn, Severely Restricted Or Not Approved by Governments: Chemicals. United Nations Publications. ISBN 978-92-1-130219-6.
- “Uragan D2” (in Czech). Lučební závody Draslovka a.s. Kolín. Archived from the original on 17 July 2015. Retrieved 7 July 2018.
- “Zyklon’ Roller Coaster Sign Is Pulled After Jewish Outcry”. The New York Times. 11 August 1993. Retrieved 5 August 2015.
- “Government: Dissent on the Wonder Drugs,” Aug. 11, 1958, [1] and Pub Policy: Pricing Fixing.
- “Public Policy: Antitrust and & Antibiotics, August 25, 1961. [2]
- Cacciapaglia and Rockman, “Proposed Drug Industry Antitrust Act–Patents, Pricing, and the Public “ 30 Geo. Wash. L. Rev. 875, 894 (1961-1962); Kennedy, “Patent and Antitrust Policy–Acquisition of Patents by Fraud or by Unfair or Deceptive Acts or Practices,” 35 George Washington Law Review 512, 531 (1966-1967) For an early discussion, see Costello, “The Tetracycline Conspiracy: Structure, Conduct and Performance in the Drug Industry,” Antitrust L. & Economic Rev. 12 (1967-1968)
- State of North Carolina v. Chas Pfizer & Co Inc. 537 F. 2d 67 (4th Cir. 1976).
- U.S. v. Pfizer,, 676 F.2d 51 (1982).
- Worobey M, Santiago ML, Keele BF, Ndjango JB, Joy JB, Labama BL, Dhed’A BD, Rambaut A, Sharp PM, Shaw GM, Hahn BH (2004). “Origin of AIDS: contaminated polio vaccine theory refuted” (PDF file, direct download 203 KB). Nature. 428 (6985): 820. Bibcode:2004Natur.428..820W. doi:10.1038/428820a. PMID 15103367. S2CID 4418410.
Note 1,13: Korber, B.et al. Science 288, 1789–1796 (2000)
- Martin B (August 2003). “Investigating the origin of AIDS: some ethical dimensions”. J Med Ethics. 29 (4): 253–256. doi:10.1136/jme.29.4.253. PMC 1733782. PMID 12930866.
- Profile, Whatisbiotechnology.org; accessed 21 April 2015.
- Dr Hilary Koprowski: Virologist who developed the first oral vaccine against polio, independent.co.uk; accessed 21 April 2015.
- The Pawel Koprowski Memorial Vacation Award was founded by Hilary Koprowski in 1958 in honor of his father.
- The Koprowski and Berland Genealogy, ancestry.com; accessed 21 April 2015.
- Mémoires of Judith Yazvina (2004)
- Biography, U.S. National Library of Medicine website; accessed 21 April 2015.
- Koprowska, Irena (1997). A Woman Wanders Through Life and Science. State University of New York Press. ISBN 9780791431771.
- Hilary Koprowski, Who Developed First Live-Virus Polio Vaccine, Dies at 96 – The New York Times, April 20, 2013.
- Plotkin, S.A., “In Memoriam: Hilary Koprowski, 1916–2013”, J. Virol., August 2013 vol. 87 no. 15, pp. 8270-8271. doi: 10.1128/JVI.01449-13. Accessed 24 May 2017.
- Hilary Koprowski, polio vaccine pioneer, dead at 96, philly.com, April 13, 2013.
- “Claude Koprowski Obituary”. 22 October 2020.
- “Nutrition Outreach Programs”.
- Zheng, Zhichao; Diaz-Arévalo, Diana; Guan, Hongbing; Zeng, Mingtao (2018-07-03). “Noninvasive vaccination against infectious diseases”. Human Vaccines & Immunotherapeutics. 14 (7): 1717–1733. doi:10.1080/21645515.2018.1461296. ISSN 2164-5515. PMC 6067898. PMID 29624470.
- “Rabies Vaccine 2”, History of Vaccines, The College of Physicians of Philadelphia (2017). Accessed 24 May 2017.
- Directory [of] PIASA Members, p. 25.
- “Honorary doctorates – Uppsala University, Sweden”.
- Award Ceremony and speeches Archived 2008-10-02 at the Wayback Machine
- “Panel nixes Congo trials as AIDS source”. Science. 258 (5083): 738–39. 1992. doi:10.1126/science.258.5083.738-d. PMID 1439779.
- “Origin of AIDS” update: Clarification Archived 2008-08-05 at the Wayback Machine, uow.edu.au, 9 December 1993, p. 39
- Brian Martin (2001) “The Politics of a Scientific Meeting: the Origin-of-AIDS Debate at the Royal Society” in Politics & the Life Sciences, pp. 119-130 online Archived 2008-08-02 at the Wayback Machine
- Hilary Koprowski (1992). “AIDS and the polio vaccine”. Science. 257 (5073): 1024, 1026–27. Bibcode:1992Sci…257.1024K. doi:10.1126/science.257.5073.1024. PMID 1509249. Archived from the original on 2008-08-21. Retrieved 2006-06-09.
- LeBrun A, Cerf J, Gelfand HM, Courtois G, Plotkin SA, Koprowski H (1960) “Vaccination with the CHAT strain of type 1 attenuated poliomyelities virus in Leopoldville, Belgian Congo 1. Description of the city, its history of poliomyelitis, and the plan of the vaccination campaign”, Bull World Health Organ. 22:203-13 online Archived 2008-10-31 at the Wayback Machine
- Plotkin SA, LeBrun A, Koprowski H (1960) “Vaccination with the CHAT strain of type 1 attenuated poliomyelitis virus in Leopoldville. Belgian Congo 2. Studies of the safety and efficacy of vaccination”, Bull World Health Organ 22:215-34 online Archived 2012-02-06 at the Wayback Machine
- Plotkin SA, LeBrun A, Courtois G, Koprowski H (1961) “Vaccination with the CHAT strain of type 1 attenuated poliomyelitis virus in Leopoldville, Congo 3. Safety and efficacy during the first 21 months of study” Bull World Health Organ 24:785-92 online Archived 2012-02-06 at the Wayback Machine
External links
- NIOSH Pocket Guide to Chemical Hazards. “#0091”. National Institute for Occupational Safety and Health (NIOSH).
- Bioassay of Calcium Cyanamide for Possible Carcinogenicity (CAS No. 156-62-7)
- “Cyanamide” . Encyclopædia Britannica. Vol. 7 (11th ed.). 1911. p. 679.
- Wyeth web site
- BASF – partial acquisition of American Cyanamid in 2000
- United States Court of Appeals for the Fourth Circuit. N°02-1235. American Cyanamid Company, Plaintiff-Appellee v. St. Louis University, Defendant-Appellant. St. Louis University (SLU) paid a $16 million Missouri state court judgment to the family of a boy who became paralyzed after receiving Orimune, an oral polio vaccine and sought contribution from American Cyanamid Company, the parent company of the vaccine manufacturer.
- Criterion Catalysts & Technologies – introduction to Criterion’s catalytic reforming (mentions spin-off from American Cyanamid and the two Shell companies)
- Guide to the American Cyanamid Company Technical Bulletins 1945–1988
- Green, Richard J.; McCarthy, Jamie (July 28, 2000). “Chemistry is Not the Science: Rudolf, Rhetoric & Reduction”. Holocaust History Project.
- Wikimedia Commons has media related to Zyklon B.
- “GenAge entry for CAT (Homo sapiens)”. Human Ageing Genomic Resources. Retrieved 2009-03-05.
- “Catalase”. MadSci FAQ. madsci.org. Retrieved 2009-03-05.
- “Catalase and oxidase test video”. Regnvm Prokaryotae. Retrieved 2009-03-05.
- “EC 1.11.1.6 – catalase”. Brenda: The Comprehensive Enzyme Information System. Retrieved 2009-03-05.
- “PeroxiBase – The peroxidase database”. Swiss Institute of Bioinformatics. Archived from the original on 2008-10-13. Retrieved 2009-03-05.
- “Catalase Procedure”. MicrobeID.com. Retrieved 2009-04-22.
- “Catalase Molecule of the Month”. Protein Data Bank. Archived from the original on 2013-05-11. Retrieved 2013-01-08.
- Overview of all the structural information available in the PDB for UniProt: P04040 (Catalase) at the PDBe-KB.
- Wikimedia Commons has media related to Hilary Koprowski.
- Hilary Koprowski (2012), Polio Vaccine. Official site. Internet Archive.
- Stacey Burling (April 14, 2013), “Hilary Koprowski, polio vaccine pioneer, dead at 96”. Philly.com, Internet Archive.
- New York Times Obituary (April 21, 2013), “Hilary Koprowski dies at 96.”
| Calcium compounds |
|---|
| Agents used in chemical warfare incapacitation riot control |
|---|
| Peroxisomal and lysosomal proteins |
|---|
| Oxidoreductases: peroxidases (EC 1.11) |
|---|
| Techniques in clinical microbiology |
|---|
- Calcium compounds
- Cyanamides
- Inorganic fertilizers
- 1907 establishments in New York (state)
- 1994 disestablishments in New Jersey
- Chemical companies established in 1907
- Chemical companies of the United States
- Companies based in Passaic County, New Jersey
- Defunct manufacturing companies based in New Jersey
- Manufacturing companies disestablished in 1994
- Pharmaceutical companies based in New Jersey
- Pharmaceutical companies disestablished in 1994
- Pharmaceutical companies established in 1907
- Wyeth
- Blood agents
- Chemical weapons
- Cyanides
- German inventions
- Infrastructure of the Holocaust
- Pesticides
- Medical lawsuits
- Genes on human chromosome 11
- EC 1.11.1
- Antioxidants
- Hemoproteins
- Enzymes
- Catalysis
- Copper enzymes
- Agricultural chemicals
- Fertilizers
- Adulteration
- Food safety
- Nitrogen
- Nitrogen cycle
- Agriculture stubs
- Livestock stubs
- Accademia Nazionale di Santa Cecilia alumn
- American immunologists
- American medical researchers
- American people of Polish-Jewish descent
- American virologists
- Chopin University of Music alumni
- Commanders of the Order of the Lion of Finland
- Grand Crosses of the Order of Merit of the Republic of Poland
- Members of the Polish Academy of Sciences
- Members of the United States National Academy of Sciences
- Polio
- Polish immunologists
- Recipients of the Legion of Honour
- Rockefeller Foundation people
- University of Warsaw alumni
- Vaccinologists




Leave a Reply