The Yolk’s On Us: Unraveling the Secrets of Vitellogenesis
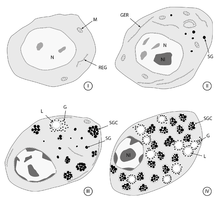
Hold onto your ovaries, folks! We’re about to dive into the wild world of Vitellogenesis – the cellular rave where eggs get their groove on!
Picture this: You’re a lonely liver cell, minding your own business, when suddenly – BAM! – you’re hit with a tsunami of estradiol. It’s like Mother Nature’s Red Bull, and honey, you’re about to grow wings! You start pumping out vitellogenin like it’s the hottest new protein on the block. And let me tell you, in the world of egg-making, vitellogenin is the VIP pass to the coolest club in town.
But wait, there’s more! This vitellogenin doesn’t just sit around looking pretty. Oh no, it hitches a ride on the bloodstream express, destination: Ovary City! It’s like Uber, but for proteins, and the surge pricing is always through the roof.
Once it arrives at the ovary, vitellogenin gets the star treatment. The oocytes roll out the red carpet (okay, it’s actually receptor-mediated endocytosis, but let’s not get too nerdy) and suck up that vitellogenin faster than a Kardashian at a photo op.
Inside the oocyte, it’s transformation time! Vitellogenin gets a makeover that would make any reality TV show jealous, turning into yolk proteins. It’s like the protein equivalent of turning into a beautiful butterfly, except instead of flying, these proteins are destined to become lunch for a developing embryo. Talk about a noble sacrifice!
But who’s pulling the strings in this protein puppet show? It’s a hormonal hullabaloo! In fish, we’ve got the hypothalamic-pituitary-gonadal axis playing conductor, with GnRH, FSH, LH, and estradiol all taking solos. It’s like an orchestra, if the orchestra was made up entirely of tiny molecules screaming “MAKE EGGS!” at the top of their lungs.
Insects, not to be outdone, have their own hormone hoedown. Juvenile hormone and 20-hydroxyecdysone are the DJs at this cellular shindig, dropping beats that make ovaries want to get down and boogie.
And let’s not forget about the nutritional nuances! The TOR signaling pathway is like the bouncer at this protein party, deciding who gets in based on how many amino acids they’re packing. Meanwhile, insulin-like peptides are running around like overexcited party planners, making sure everything’s in order.
But the real MVPs? MicroRNAs. These tiny troublemakers are like the gossip columnists of the cellular world, regulating everything with a whisper and a wink. They’re small, they’re sneaky, and they’ve got more influence than you’d believe.
So, there you have it, folks! Vitellogenesis: the biological bash where proteins party, hormones harmonize, and eggs get eggsactly what they need to bring the next generation into the world. It’s a miracle of nature, a triumph of evolution, and proof that even on a cellular level, life finds a way to be absolutely, undeniably, egg-cellent!
Other Notes (Wikipedia)
Vitellogenesis is the process of yolk protein formation in the oocytes during sexual maturation.
- Wallace RA (1985). “Vitellogenesis and Oocyte Growth in Nonmammalian Vertebrates”. In Browder LW (ed.). Oogenesis. Boston, MA: Springer US. pp. 127–177. doi:10.1007/978-1-4615-6814-8_3. ISBN 978-1-4615-6816-2.
The term vitellogenesis comes from the Latin vitellus (“egg yolk”). Yolk proteins, such as Lipovitellin and Phosvitin, provides maturing oocytes with the metabolic energy required for development. Vitellogenins are the precursor proteins that lead to yolk protein accumulation in the oocyte.
In vertebrates, estrogen and vitellogenin production have a positive correlation. When estrogen production in the ovary is increased via the activation of the hypothalmo-pituitary axis it leads to heightened vitellogenin production in the liver.
- Ho SM (1987). “Endocrinology of Vitellogenesis”. In Norris DG, Jones RE (eds.). Hormones and Reproduction in Fishes, Amphibians, and Reptiles. Boston, MA: Springer US. pp. 145–169. doi:10.1007/978-1-4613-1869-9_6. ISBN 978-1-4612-9042-1.
Vitellogenin production in the liver is the first step of vitellogenesis. Once Vitellogenins are released into the blood stream where they are then transported to the growing oocyte where they lead to yolk protein production. The transport of vitellogenins into the maturing oocyte is done via endocytosis mediated by a receptor which is a low-density lipoprotein receptor (LDLR). Yolk is a lipoprotein composed of proteins, phospholipids and neutral fats along with a small amount of glycogen. The yolk is synthesised in the liver of the female parent in soluble form. Through circulation it is transported to the follicle cells that surround the maturing ovum, and is deposited in the form of yolk platelets and granules in the ooplasm. The mitochondria and Golgi complex are said to bring about the conversion of the soluble form of yolk into insoluble granules or platelets.
The two hormones responsible for vitellogenesis stimulation in insects are sesquiterpenoid juvenile hormone (JH) and ecdysteroid 20-hydroxyecdysone (E20). More recent studies are showing the importance of miRNA in vitellogenesis stimulation as well. The pathways that these hormones regulate is largely dependent on the evolutionary growth of the insect species. Together, JH, E20, and miRNA help synthesize vitellogenins within the fat body. JH uses a JH Methoprene tolerant /Taiman receptor complex that is regulated by JH to synthesis vitellogenins in the fat body.
- Wu Z, Yang L, He Q, Zhou S (2021-01-28). “Regulatory Mechanisms of Vitellogenesis in Insects”. Frontiers in Cell and Developmental Biology. 8: 593613. doi:10.3389/fcell.2020.593613. PMC 7901893. PMID 33634094.
In cockroaches, for example, vitellogenesis can be stimulated by injection of juvenile hormone into immature females and mature males. In mosquitoes infected with Plasmodium, vitellogenesis may be manipulated by the parasites to reduce fecundity, thereby preserving nutrition in the infected individual.
- Hurd H (2003). “Manipulation of medically important insect vectors by their parasites”. Annual Review of Entomology. 48 (1): 141–161. doi:10.1146/annurev.ento.48.091801.112722. PMID 12414739.
| I. Holoblastic (complete) cleavage | II. Meroblastic (incomplete) cleavage |
|---|---|
| A. Isolecithal (sparse, evenly distributed yolk) 1. Radial cleavage (echinoderms, hemichordates, amphioxus) 2. Spiral cleavage (annelids, most mollusks, flatworms) 3. Bilateral cleavage (tunicates) 4. Rotational cleavage (placental mammals, nematodes, marsupials [?]) B. Mesolecithal (moderate vegetal yolk disposition) Displaced radial cleavage (amphibians, some fish [the lampreys, gars and bowfins) | A. Telolecithal (dense yolk throughout most of cell) 1. Bilateral cleavage (cephalopod molluscs) 2. Discoidal cleavage (some fish [the hagfishes, chondrichthyans and most teleosts], sauropsids [reptiles and birds], monotremes) B. Centrolecithal (yolk in center of egg) Superficial cleavage (most insects) |
Gilbert SF (2003). Developmental biology (7th ed.). Sinauer. p. 214. ISBN 0-87893-258-5.
Kardong KV (20106). Vertebrates: Comparative Anatomy, Function, Evolution (4th ed.). McGraw-Hill. pp. 158–64.2
References
- Greani S, Quilichini Y, Marchand B (2016). “Ultrastructural study of vitellogenesis and oogenesis of Crepidostomum metoecus (Digenea, Allocreadiidae), intestinal parasite of Salmo trutta (Pisces, Teleostei)”. Parasite. 23: 47. doi:10.1051/parasite/2016057. PMC 5112763. PMID 27845028.
- Wallace RA (1985). “Vitellogenesis and Oocyte Growth in Nonmammalian Vertebrates”. In Browder LW (ed.). Oogenesis. Boston, MA: Springer US. pp. 127–177. doi:10.1007/978-1-4615-6814-8_3. ISBN 978-1-4615-6816-2.
- Ho SM (1987). “Endocrinology of Vitellogenesis”. In Norris DG, Jones RE (eds.). Hormones and Reproduction in Fishes, Amphibians, and Reptiles. Boston, MA: Springer US. pp. 145–169. doi:10.1007/978-1-4613-1869-9_6. ISBN 978-1-4612-9042-1.
- Wu Z, Yang L, He Q, Zhou S (2021-01-28). “Regulatory Mechanisms of Vitellogenesis in Insects”. Frontiers in Cell and Developmental Biology. 8: 593613. doi:10.3389/fcell.2020.593613. PMC 7901893. PMID 33634094.
- Hurd H (2003). “Manipulation of medically important insect vectors by their parasites”. Annual Review of Entomology. 48 (1): 141–161. doi:10.1146/annurev.ento.48.091801.112722. PMID 12414739.
- Gilbert SF (2003). Developmental biology (7th ed.). Sinauer. p. 214. ISBN 0-87893-258-5.
- Kardong KV (2006). Vertebrates: Comparative Anatomy, Function, Evolution (4th ed.). McGraw-Hill. pp. 158–64.


Leave a Reply