Paracetamol/acetaminophen (N-Acetyl-p-Aminophenol; APAP) is the preferred analgesic for pain relief and fever during pregnancy. It has therefore caused concern that several studies have reported that prenatal exposure to APAP results in developmental alterations in both the reproductive tract and the brain. Genitals and nervous system of male mammals are actively masculinised during foetal development and early postnatal life by the combined actions of prostaglandins and androgens, resulting in the male-typical reproductive behaviour seen in adulthood. Both androgens and prostaglandins are known to be inhibited by APAP. Through intrauterine exposure experiments in C57BL/6 mice, we found that exposure to APAP decreased neuronal number in the sexually dimorphic nucleus (SDN) of the preoptic area (POA) in the anterior hypothalamus of male adult offspring. Likewise, exposure to the environmental pollutant and precursor of APAP, aniline, resulted in a similar reduction. Decrease in neuronal number in the SDN-POA is associated with reductions in male sexual behaviour. Consistent with the changes, male mice exposed in uteri to APAP exhibited changes in urinary marking behaviour as adults and had a less aggressive territorial display towards intruders of the same gender. Additionally, exposed males had reduced intromissions and ejaculations during mating with females in oestrus. Together, these data suggest that prenatal exposure to APAP may impair male sexual behaviour in adulthood by disrupting the sexual neurobehavioral programming. These findings add to the growing body of evidence suggesting the need to limit the widespread exposure and use of APAP by pregnant women.
Hay-Schmidt A, Finkielman OTE, Jensen BAH, Høgsbro CF, Bak Holm J, Johansen KH, Jensen TK, Andrade AM, Swan SH, Bornehag CG, Brunak S, Jegou B, Kristiansen K, Kristensen DM. Prenatal exposure to paracetamol/acetaminophen and precursor aniline impairs masculinisation of male brain and behaviour. Reproduction. 2017 Aug;154(2):145-152. doi: 10.1530/REP-17-0165. Epub 2017 May 30. PMID: 28559473. © 2017 Society for Reproduction and Fertility.
Aniline (Wikipedia)
Aniline (from Portugueseanil ‘indigo shrub’, and -ine indicating a derived substance) is an organic compound with the formula C6H5NH2. Consisting of a phenyl group (−C6H5) attached to an amino group (−NH2), aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.
Aniline is predominantly used for the preparation of methylenedianiline and related compounds by condensation with formaldehyde. The diamines are condensed with phosgene to give methylene diphenyl diisocyanate, a precursor to urethane polymers.
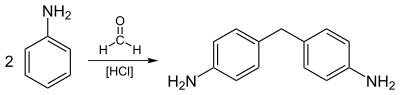
Other uses include rubber processing chemicals (9%), herbicides (2%), and dyes and pigments (2%). As additives to rubber, aniline derivatives such as phenylenediamines and diphenylamine, are antioxidants. Illustrative of the drugs prepared from aniline is paracetamol (acetaminophen, Tylenol). The principal use of aniline in the dye industry is as a precursor to indigo, the blue of blue jeans.

History
Aniline was first isolated in 1826 by Otto Unverdorben by destructive distillation of indigo. He called it Crystallin. In 1834, Friedlieb Runge isolated a substance from coal tar that turned a beautiful blue color when treated with chloride of lime. He named it kyanol or cyanol. In 1840, Carl Julius Fritzsche (1808–1871) treated indigo with caustic potash and obtained an oil that he named aniline, after an indigo-yielding plant, anil (Indigofera suffruticosa). In 1842, Nikolay Nikolaevich Zinin reduced nitrobenzene and obtained a base that he named benzidam. In 1843, August Wilhelm von Hofmann showed that these were all the same substance, known thereafter as phenylamine or aniline.
Synthetic dye industry
In 1856, while trying to synthesise quinine, von Hofmann‘s student William Henry Perkin discovered mauveine. Mauveine quickly became a commercial dye. Other synthetic dyes followed, such as fuchsin, safranin, and induline. At the time of mauveine’s discovery, aniline was expensive. Soon thereafter, applying a method reported in 1854 by Antoine Béchamp, it was prepared “by the ton”. The Béchamp reduction enabled the evolution of a massive dye industry in Germany. Today, the name of BASF, originally Badische Anilin- und Soda-Fabrik (English: Baden Aniline and Soda Factory), now the largest chemical supplier, echoes the legacy of the synthetic dye industry, built via aniline dyes and extended via the related azo dyes. The first azo dye was aniline yellow.
Developments in medicine
In the late 19th century, derivatives of aniline such as acetanilide and phenacetin emerged as analgesic drugs, with their cardiac-suppressive side effects often countered with caffeine.[32] During the first decade of the 20th century, while trying to modify synthetic dyes to treat African sleeping sickness, Paul Ehrlich – who had coined the term chemotherapy for his magic bullet approach to medicine – failed and switched to modifying Béchamp‘s atoxyl, the first organic arsenical drug, and serendipitously obtained a treatment for syphilis – salvarsan – the first successful chemotherapy agent. Salvarsan’s targeted microorganism, not yet recognized as a bacterium, was still thought to be a parasite, and medical bacteriologists, believing that bacteria were not susceptible to the chemotherapeutic approach, overlooked Alexander Fleming‘s report in 1928 on the effects of penicillin.
In 1932, Bayer sought medical applications of its dyes. Gerhard Domagk identified as an antibacterial a red azo dye, introduced in 1935 as the first antibacterial drug, prontosil, soon found at Pasteur Institute to be a prodrug degraded in vivo into sulfanilamide – a colorless intermediate for many, highly colorfast azo dyes – already with an expired patent, synthesized in 1908 in Vienna by the researcher Paul Gelmo for his doctoral research. By the 1940s, over 500 related sulfa drugs were produced. Medications in high demand during World War II (1939–45), these first miracle drugs, chemotherapy of wide effectiveness, propelled the American pharmaceutics industry. In 1939, at Oxford University, seeking an alternative to sulfa drugs, Howard Florey developed Fleming’s penicillin into the first systemic antibiotic drug, penicillin G. (Gramicidin, developed by René Dubos at Rockefeller Institute in 1939, was the first antibiotic, yet its toxicity restricted it to topical use.) After World War II, Cornelius P. Rhoads introduced the chemotherapeutic approach to cancer treatment.
Rocket fuel
Some early American rockets, such as the Aerobee and WAC Corporal, used a mixture of aniline and furfuryl alcohol as a fuel, with nitric acid as an oxidizer. The combination is hypergolic, igniting on contact between fuel and oxidizer. It is also dense, and can be stored for extended periods. Aniline was later replaced by hydrazine.
Toxicology and testing
Aniline is toxic by inhalation of the vapour, ingestion, or percutaneous absorption. The IARC lists it in Group 3 (not classifiable as to its carcinogenicity to humans) due to the limited and contradictory data available. The early manufacture of aniline resulted in increased incidents of bladder cancer, but these effects are now attributed to naphthylamines, not anilines.
Aniline has been implicated as one possible cause of forest dieback.
Many methods exist for the detection of aniline.
Oxidative DNA damage
Exposure of rats to aniline can elicit a response that is toxic to the spleen, including a tumorigenic response. Rats exposed to aniline in drinking water, showed a significant increase in oxidative DNA damage to the spleen, detected as a 2.8-fold increase in 8-hydroxy-2′-deoxyguanosine (8-OHdG) in their DNA. Although the base excision repair pathway was also activated, its activity was not sufficient to prevent the accumulation of 8-OHdG. The accumulation of oxidative DNA damages in the spleen following exposure to aniline may increase mutagenic events that underlie tumorigenesis.
- “aniline | Etymology, origin and meaning of aniline by etymonline”. www.etymonline.com. Retrieved 2022-02-15.
- Yoshikawa N, Sarower MG, Abe H (2016). “Alanine Racemase and D-Amino Acid Oxidase in Aquatic Animals”. In Yoshimura T, Nishikawa T, Homma H (eds.). D-Amino Acids: Physiology, Metabolism, and Application. Springer Japan. pp. 269–282. ISBN 978-4-431-56077-7.
- “Aniline”. The Chemical Market Reporter. Archived from the original on 2002-02-19. Retrieved 2007-12-21.
- Otto Unverdorben (1826). “Ueber das Verhalten der organischen Körper in höheren Temperaturen” [On the behaviour of organic substances at high temperatures]. Annalen der Physik und Chemie. 8 (11): 397–410. Bibcode:1826AnP….84..397U. doi:10.1002/andp.18260841109.
- F. F. Runge (1834) “Ueber einige Produkte der Steinkohlendestillation” (On some products of coal distillation), Annalen der Physik und Chemie, 31: 65–77 (see page 65), 513–524; and 32: 308–332 (see page 331).
- J. Fritzsche (1840) “Ueber das Anilin, ein neues Zersetzungsproduct des Indigo” (On aniline, a new decomposition product of indigo), Bulletin Scientifique [publié par l’Académie Impériale des Sciences de Saint-Petersbourg], 7 (12): 161–165. Reprinted in:
- J. Fritzsche (1840) “Ueber das Anilin, ein neues Zersetzungsproduct des Indigo”, Justus Liebigs Annalen der Chemie, 36 (1): 84–90.
- J. Fritzsche (1840) “Ueber das Anilin, ein neues Zersetzungsproduct des Indigo”, Journal für praktische Chemie, 20: 453–457. In a postscript to this article, Erdmann (one of the journal’s editors) argues that aniline and the “cristallin”, which was found by Unverdorben in 1826, are the same substance; see pages 457–459.
- synonym I anil, ultimately from Sanskrit “nīla”, dark-blue.
- N. Zinin (1842). “Beschreibung einiger neuer organischer Basen, dargestellt durch die Einwirkung des Schwefelwasserstoffes auf Verbindungen der Kohlenwasserstoffe mit Untersalpetersäure” (Description of some new organic bases, produced by the action of hydrogen sulfide on compounds of hydrocarbons and hyponitric acid [H2N2O3]), Bulletin Scientifique [publié par l’Académie Impériale des Sciences de Saint-Petersbourg], 10 (18): 272–285. Reprinted in: N. Zinin (1842) “Beschreibung einiger neuer organischer Basen, dargestellt durch die Einwirkung des Schwefelwasserstoffes auf Verbindungen der Kohlenwasserstoffe mit Untersalpetersäure”, Journal für praktische Chemie, 27 (1): 140–153. Benzidam is named on page 150. Fritzsche, Zinin’s colleague, soon recognized that “benzidam” was actually aniline. See: Fritzsche (1842) Bulletin Scientifique, 10: 352. Reprinted as a postscript to Zinin’s article in: J. Fritzsche (1842) “Bemerkung zu vorstehender Abhandlung des Hrn. Zinin” (Comment on the preceding article by Mr. Zinin), Journal für praktische Chemie, 27 (1): 153.
See also: (Anon.) (1842) “Organische Salzbasen, aus Nitronaphtalose und Nitrobenzid mittelst Schwefelwasserstoff entstehend” (Organic bases originating from nitronaphthalene and nitrobenzene via hydrogen sulfide), Annalen der Chemie und Pharmacie, 44: 283–287. - August Wilhelm Hofmann (1843) “Chemische Untersuchung der organischen Basen im Steinkohlen-Theeröl” (Chemical investigation of organic bases in coal tar oil), Annalen der Chemie und Pharmacie, 47: 37–87. On page 48, Hofmann argues that krystallin, kyanol, benzidam, and aniline are identical.
- A. Béchamp (1854) “De l’action des protosels de fer sur la nitronaphtaline et la nitrobenzine. Nouvelle méthode de formation des bases organiques artificielles de Zinin” (On the action of iron protosalts on nitronaphthaline and nitrobenzene. New method of forming Zinin’s synthetic organic bases.), Annales de Chemie et de Physique, 3rd series, 42: 186 – 196. (Note: In the case of a metal having two or more distinct oxides (e.g., iron), a “protosalt” is an obsolete term for a salt that is obtained from the oxide containing the lowest proportion of oxygen to metal; e.g., in the case of iron, which has two oxides – iron (II) oxide (FeO) and iron (III) oxide (Fe2O3) – FeO is the “protoxide” from which protosalts can be made. See: Wiktionary: protosalt.)
- Perkin, William Henry. 1861-06-08. “Proceedings of Chemical Societies: Chemical Society, Thursday, May 16, 1861”. The Chemical News and Journal of Industrial Science. Retrieved on 2007-09-24.
- Auerbach G, “Azo and naphthol dyes”, Textile Colorist, 1880 May;2(17):137-9, p 138.
- Wilcox RW, “The treatment of influenza in adults”, Medical News, 1900 Dec 15;77():931-2, p 932.
- D J Th Wagener, The History of Oncology (Houten: Springer, 2009), pp 150–1.
- John E Lesch, The First Miracle Drugs: How the Sulfa Drugs Transformed Medicine (New York: Oxford University Press, 2007), pp 202–3.
- “Medicine: Spoils of War”. Time. 15 May 1950. Archived from the original on 24 June 2013. Retrieved 20 November 2020.
- Brian Burnell. 2016. http://www.nuclear-weapons.info/cde.htm#Corporal SSM
- Muir, GD (ed.) 1971, Hazards in the Chemical Laboratory, The Royal Institute of Chemistry, London.
- The Merck Index. 10th ed. (1983), p.96, Rahway: Merck & Co.
- Krahl-Urban, B., Papke, H.E., Peters, K. (1988) Forest Decline: Cause-Effect Research in the United States of North America and Federal Republic of Germany. Germany: Assessment Group for Biology, Ecology and Energy of the Julich Nuclear Research Center.
- Basic Analytical Toxicology (1995), R. J. Flanagan, S. S. Brown, F. A. de Wolff, R. A. Braithwaite, B. Widdop: World Health Organization
- Ma, Huaxian; Wang, Jianling; Abdel-Rahman, Sherif Z.; Boor, Paul J.; Khan, M. Firoze (2008). “Oxidative DNA damage and its repair in rat spleen following subchronic exposure to aniline”. Toxicology and Applied Pharmacology. 233 (2): 247–253. doi:10.1016/j.taap.2008.08.010. PMC 2614128. PMID 18793663.