Glycodelin (GD) also known as human placental protein-14 (PP-14) progestogen-associated endometrial protein (PAEP) or pregnancy-associated endometrial alpha-2 globulin is a glycoprotein that inhibits cell immune function and plays an essential role in the pregnancy process. In humans is encoded by the PAEP gene.
- “PAEP – Glycodelin precursor – Homo sapiens (Human) – PAEP gene & protein”. www.uniprot.org.
- Wang, Ping; Libho, Zhu; Xinmei, Zhang (December 2013). “The role of Placental Protein 14 in the Pathogenesis of Endometrosis”. Reproductive Sciences. 20 (12): 1465–1470. doi:10.1177/1933719113488452. ISSN 0077-8923. PMC 3817670. PMID 23670949.
Human endometrium synthesizes several proteins under the influence of progesterone. Of these proteins, glycodelin is of particular interest. It is synthesized by the endometrial glands in the luteal phase of menstrual cycle.
- SEPPÄLÄ, MARKKU; JULKUNEN, MERVI; KOSKIMIES, AARNE; LAATIKAINEN, TIMO; STENMAN, ULF–HÅKAN; HUHTALA, MARJA-LIISA (October 1988). “Proteins of the Human Endometrium”. Annals of the New York Academy of Sciences. 541 (1): 432–444. Bibcode:1988NYASA.541..432S. doi:10.1111/j.1749-6632.1988.tb22280.x. ISSN 0077-8923. PMID 3195927. S2CID 222073546.
The temporal and spatial expression of GD in the female reproductive tract combined with its biological activities suggest that this glycoprotein probably plays an essential physiological role in the regulation of fertilization, implantation and maintenance of pregnancy.
- Dutta, Binita; Mukhopadhyay, Debaditya; Roy, Nita; Das, Goutam; Karande, Anjali A. (December 1998). “Cloning, Expression, Purification, and Immunocharacterization of Placental Protein-14”. Protein Expression and Purification. 14 (3): 327–334. doi:10.1006/prep.1998.0961. ISSN 1046-5928. PMID 9882566.
- Julkunen, M.; Seppala, M.; Janne, O. A. (1988-12-01). “Complete amino acid sequence of human placental protein 14: a progesterone-regulated uterine protein homologous to beta-lactoglobulins”. Proceedings of the National Academy of Sciences. 85 (23): 8845–8849. Bibcode:1988PNAS…85.8845J. doi:10.1073/pnas.85.23.8845. ISSN 0027-8424. PMC 282603. PMID 3194393.
Structure
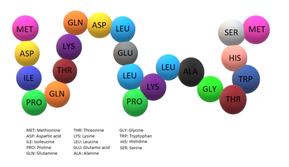
Glycodelin is codified by 180 amino acid but it is thought that 18 of these are supposed signals peptides. The molecular weight of GD is 20,555, while its mature form is estimated to weigh 18,787. It is encoded by a 1-kilobase-pair mRNA that is expressed in human secretory endometrium and decidua but not in postmenopausal endometrium, placenta, liver, kidney, and adrenals. The four cysteinyl residues (positions 66, 106, 119, and 160) responsible for intramolecular disulfide bridges in lactoglobulins are all conserved in GD. Southern blot analysis of human DNA suggested that GD gene sequences compass some 20 kilobase pairs of the human genomic DNA.
- Julkunen, M.; Seppala, M.; Janne, O. A. (1988-12-01). “Complete amino acid sequence of human placental protein 14: a progesterone-regulated uterine protein homologous to beta-lactoglobulins”. Proceedings of the National Academy of Sciences. 85 (23): 8845–8849. Bibcode:1988PNAS…85.8845J. doi:10.1073/pnas.85.23.8845. ISSN 0027-8424. PMC 282603. PMID 3194393.
N-terminal amino acid sequence
The N-terminal amino acid sequence of glycodelin is M D I P Q T K Q D L E L P K L A G T W H S M. This sequence can be compared with horse, sheep, goat, bovine and buffalo beta-lactoglobulin. For example, there are 13 identities out of 22 possible matches with horse beta-lactoglobulin.
β-Lactoglobulin (BLG) is the major whey protein of cow and sheep‘s milk (~3 g/L), and is also present in many other mammalian species; a notable exception being humans. Its structure, properties and biological role have been reviewed many times. BLG is considered to be a milk allergen. The major protein in whey is β-lactoglobulin, followed by α-lactalbumin (β-lactoglobulin ≈ 65%, α-lactalbumin ≈ 25%, serum albumin ≈ 8%, other ≈ 2%). β-lactoglobulin is a lipocalin protein, and can bind many hydrophobic molecules, suggesting a role in their transport. β-lactoglobulin has also been shown to be able to bind iron via siderophores and thus might have a role in combating pathogens. Upon ingestion BLG is able to shuttle complexed iron into human immune cells, thereby providing micronutrition to these cells and participating in immune tolerance. A homologue of β-lactoglobulin is lacking in human breast milk.
- Xiang L, Melton L, Leung KH (2019). “Interactions of β-Lactoglobulin With Small Molecules”. In Varelis P, Melton L, Shahidi F (eds.). Encyclopedia of Food Chemistry. Vol. 2. Elsevier. pp. 560–565. doi:10.1016/B978-0-08-100596-5.21488-1. ISBN 9780128140451. S2CID 90712856.
- Sawyer L (1992). “Beta-lactoglobulin”. In Fox PF, McSweeney PL (eds.). Advanced Dairy Chemistry: 1. Proteins. Elsevier Applied Science. pp. 141–190. doi:10.1007/978-1-4419-8602-3_7. ISBN 978-1-4419-8602-3.
- Sawyer L, Kontopidis G (October 2000). “The core lipocalin, bovine beta-lactoglobulin”. Biochimica et Biophysica Acta (BBA) – Protein Structure and Molecular Enzymology. 1482 (1–2): 136–48. doi:10.1016/s0167-4838(00)00160-6. PMID 11058756.
- Kontopidis G, Holt C, Sawyer L (April 2004). “Invited review: beta-lactoglobulin: binding properties, structure, and function”. Journal of Dairy Science. 87 (4): 785–96. doi:10.3168/jds.S0022-0302(04)73222-1. PMID 15259212.
- listed in Annex IIIa of Directive 2000/13/EC
- Wei, Jingwei; Wagner, Stefan; Maclean, Paul; Brophy, Brigid; Cole, Sally; Smolenski, Grant; Carlson, Dan F.; Fahrenkrug, Scott C.; Wells, David N.; Laible, Götz (2018-05-16). “Cattle with a precise, zygote-mediated deletion safely eliminate the major milk allergen beta-lactoglobulin”. Scientific Reports. 8 (1): 7661. Bibcode:2018NatSR…8.7661W. doi:10.1038/s41598-018-25654-8. ISSN 2045-2322. PMC 5955954. PMID 29769555.
- Roth-Walter F, Pacios LF, Gomez-Casado C, Hofstetter G, Roth GA, Singer J, et al. (2014-01-01). “The major cow milk allergen Bos d 5 manipulates T-helper cells depending on its load with siderophore-bound iron”. PLOS ONE. 9 (8): e104803. Bibcode:2014PLoSO…9j4803R. doi:10.1371/journal.pone.0104803. PMC 4130594. PMID 25117976.
- Roth-Walter F, Afify SM, Pacios LF, Blokhuis BR, Redegeld F, Regner A, et al. (January 2021). “Cow’s milk protein β-lactoglobulin confers resilience against allergy by targeting complexed iron into immune cells”. The Journal of Allergy and Clinical Immunology. 147 (1): 321–334.e4. doi:10.1016/j.jaci.2020.05.023. hdl:10261/272213. PMID 32485264. S2CID 219285403
- Afify SM, Pali-Schöll I, Hufnagl K, Hofstetter G, El-Bassuoni MA, Roth-Walter F, Jensen-Jarolim E (2021). “Bovine Holo-Beta-Lactoglobulin Cross-Protects Against Pollen Allergies in an Innate Manner in BALB/c Mice: Potential Model for the Farm Effect”. Frontiers in Immunology. 12: 611474. doi:10.3389/fimmu.2021.611474. PMC 7977286. PMID 33746954.
- Fiocchi A, Brozek J, Schünemann H, Bahna SL, von Berg A, Beyer K, et al. (April 2010). “World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines”. The World Allergy Organization Journal. 3 (4): 57–161. doi:10.1097/WOX.0b013e3181defeb9. PMC 3488907. PMID 23268426.
β-lactoglobulin is of direct interest to the food industry since its properties can variously be advantageous or disadvantageous in dairy products and processing. Though β-lactoglobulin is considered a major allergen, the protective impact of the consumption of raw milk has been shown to dependent on the protein-content of the whey fraction and thus of β-lactoglobulin. This great contrast, on the one hand an allergen and on the other protective, has now been linked with its ability to carry micronutrient. When β-lactoglobulin carried micronutrient it acted tolerogenic and protected against allergy development. However, when the loading was missing, it turned into an allergen. Laboratory polymerization of β-lactoglobulin by microbial transglutaminase reduces its allergenicity in children and adults with an IgE-mediated cow’s milk allergy. Bovine β-lactoglobulin is a relatively small protein of 162 residues, with an 18.4 kDa. In physiological conditions it is predominantly dimeric, but dissociates to a monomer below about pH 3, preserving its native state as determined by using NMR. Conversely, β-lactoglobulin also occurs in tetrameric, octameric and other multimeric aggregation forms under a variety of natural conditions. In 2018 it was announced that genetically modified cows were grown. The cows had their β-Lactoglobulin producing genes removed by a zygote-mediated deletion process. See also: Caseine
- Xiang L, Melton L, Leung KH (2019). “Interactions of β-Lactoglobulin With Small Molecules”. In Varelis P, Melton L, Shahidi F (eds.). Encyclopedia of Food Chemistry. Vol. 2. Elsevier. pp. 560–565. doi:10.1016/B978-0-08-100596-5.21488-1. ISBN 9780128140451. S2CID 90712856.
- Sawyer L (1992). “Beta-lactoglobulin”. In Fox PF, McSweeney PL (eds.). Advanced Dairy Chemistry: 1. Proteins. Elsevier Applied Science. pp. 141–190. doi:10.1007/978-1-4419-8602-3_7. ISBN 978-1-4419-8602-3.
- Sawyer L, Kontopidis G (October 2000). “The core lipocalin, bovine beta-lactoglobulin”. Biochimica et Biophysica Acta (BBA) – Protein Structure and Molecular Enzymology. 1482 (1–2): 136–48. doi:10.1016/s0167-4838(00)00160-6. PMID 11058756.
- Kontopidis G, Holt C, Sawyer L (April 2004). “Invited review: beta-lactoglobulin: binding properties, structure, and function”. Journal of Dairy Science. 87 (4): 785–96. doi:10.3168/jds.S0022-0302(04)73222-1. PMID 15259212.
- listed in Annex IIIa of Directive 2000/13/EC
- Wei, Jingwei; Wagner, Stefan; Maclean, Paul; Brophy, Brigid; Cole, Sally; Smolenski, Grant; Carlson, Dan F.; Fahrenkrug, Scott C.; Wells, David N.; Laible, Götz (2018-05-16). “Cattle with a precise, zygote-mediated deletion safely eliminate the major milk allergen beta-lactoglobulin”. Scientific Reports. 8 (1): 7661. Bibcode:2018NatSR…8.7661W. doi:10.1038/s41598-018-25654-8. ISSN 2045-2322. PMC 5955954. PMID 29769555.
- Roth-Walter F, Pacios LF, Gomez-Casado C, Hofstetter G, Roth GA, Singer J, et al. (2014-01-01). “The major cow milk allergen Bos d 5 manipulates T-helper cells depending on its load with siderophore-bound iron”. PLOS ONE. 9 (8): e104803. Bibcode:2014PLoSO…9j4803R. doi:10.1371/journal.pone.0104803. PMC 4130594. PMID 25117976.
- Roth-Walter F, Afify SM, Pacios LF, Blokhuis BR, Redegeld F, Regner A, et al. (January 2021). “Cow’s milk protein β-lactoglobulin confers resilience against allergy by targeting complexed iron into immune cells”. The Journal of Allergy and Clinical Immunology. 147 (1): 321–334.e4. doi:10.1016/j.jaci.2020.05.023. PMID 32485264. S2CID 219285403.
- Afify SM, Pali-Schöll I, Hufnagl K, Hofstetter G, El-Bassuoni MA, Roth-Walter F, Jensen-Jarolim E (2021). “Bovine Holo-Beta-Lactoglobulin Cross-Protects Against Pollen Allergies in an Innate Manner in BALB/c Mice: Potential Model for the Farm Effect”. Frontiers in Immunology. 12: 611474. doi:10.3389/fimmu.2021.611474. PMC 7977286. PMID 33746954.
- Fiocchi A, Brozek J, Schünemann H, Bahna SL, von Berg A, Beyer K, et al. (April 2010). “World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines”. The World Allergy Organization Journal. 3 (4): 57–161. doi:10.1097/WOX.0b013e3181defeb9. PMC 3488907. PMID 23268426.
- PDB: 3BLG; Qin BY, Bewley MC, Creamer LK, Baker HM, Baker EN, Jameson GB (October 1998). “Structural basis of the Tanford transition of bovine beta-lactoglobulin”. Biochemistry. 37 (40): 14014–23. doi:10.1021/bi981016t. PMID 9760236.
- Jost R (1993). “Functional characteristics of dairy proteins”. Trends in Food Science & Technology. 4 (9): 283–288. doi:10.1016/0924-2244(93)90071-H.
- Uhrínová S, Smith MH, Jameson GB, Uhrín D, Sawyer L, Barlow PN (April 2000). “Structural changes accompanying pH-induced dissociation of the beta-lactoglobulin dimer”. Biochemistry. 39 (13): 3565–74. doi:10.1021/bi992629o. PMID 10736155.
- Timasheff SN, Townend R (1964). “Structure of the β-Lactoglobulin Tetramer”. Nature. 203 (4944): 517–519. Bibcode:1964Natur.203..517T. doi:10.1038/203517a0. S2CID 4190604.
- Gottschalk M, Nilsson H, Roos H, Halle B (November 2003). “Protein self-association in solution: the bovine beta -lactoglobulin dimer and octamer”. Protein Science. 12 (11): 2404–11. doi:10.1110/ps.0305903. PMC 2366967. PMID 14573854.
- Rizzuti B, Zappone B, De Santo MP, Guzzi R (January 2010). “Native beta-lactoglobulin self-assembles into a hexagonal columnar phase on a solid surface”. Langmuir. 26 (2): 1090–5. doi:10.1021/la902464f. PMID 19877696.
- Bromley EH, Krebs MR, Donald AM (2005). “Aggregation across the length-scales in beta-lactoglobulin”. Faraday Discussions. 128: 13–27. doi:10.1039/b403014a. PMID 15658764.
- Kuwajima K, Yamaya H, Sugai S (December 1996). “The burst-phase intermediate in the refolding of beta-lactoglobulin studied by stopped-flow circular dichroism and absorption spectroscopy”. Journal of Molecular Biology. 264 (4): 806–22. doi:10.1006/jmbi.1996.0678. PMID 8980687.
- Loss G, Apprich S, Waser M, Kneifel W, Genuneit J, Büchele G, et al. (October 2011). “The protective effect of farm milk consumption on childhood asthma and atopy: the GABRIELA study”. The Journal of Allergy and Clinical Immunology. 128 (4): 766–773.e4. doi:10.1016/j.jaci.2011.07.048. hdl:1874/407013. PMID 21875744.
- Roth-Walter F, Afify SM, Pacios LF, Blokhuis BR, Redegeld F, Regner A, et al. (January 2021). “Cow’s milk protein β-lactoglobulin confers resilience against allergy by targeting complexed iron into immune cells”. The Journal of Allergy and Clinical Immunology. 147 (1): 321–334.e4. doi:10.1016/j.jaci.2020.05.023. PMID 32485264.
- Hufnagl K, Ghosh D, Wagner S, Fiocchi A, Dahdah L, Bianchini R, et al. (January 2018). “Retinoic acid prevents immunogenicity of milk lipocalin Bos d 5 through binding to its immunodominant T-cell epitope”. Scientific Reports. 8 (1): 1598. Bibcode:2018NatSR…8.1598H. doi:10.1038/s41598-018-19883-0. PMC 5785490. PMID 29371615.
- Afify SM, Pali-Schöll I, Hufnagl K, Hofstetter G, El-Bassuoni MA, Roth-Walter F, Jensen-Jarolim E (2021). “Bovine Holo-Beta-Lactoglobulin Cross-Protects Against Pollen Allergies in an Innate Manner in BALB/c Mice: Potential Model for the Farm Effect”. Frontiers in Immunology. 12: 611474. doi:10.3389/fimmu.2021.611474. PMC 7977286. PMID 33746954.
- Olivier CE, Lima RP, Pinto DG, Santos RA, Silva GK, Lorena SL, et al. (October 2012). “In search of a tolerance-induction strategy for cow’s milk allergies: significant reduction of beta-lactoglobulin allergenicity via transglutaminase/cysteine polymerization”. Clinics. 67 (10): 1171–9. doi:10.6061/clinics/2012(10)09. PMC 3460020. PMID 23070344.
PAEP gene
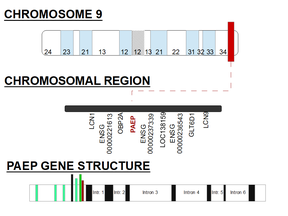
This gene is a member of the kernel lipocalin superfamily whose members share relatively low sequence similarity but have highly conserved exon-intron structure and three-dimensional protein folding. The PAEP gene is clustered on the long arm of chromosome 9 and encodes for GD protein. It is mainly expressed in 60 organs, but reaches its highest expression level in the decidua (defined as thick layer of modified mucous membrane which lines the uterus during pregnancy and is shed with the afterbirth, also modified mucosal lining of the uterus (that is, modified endometrium) that forms every month, in preparation for pregnancy).
- “PAEP progestagen associated endometrial protein [Homo sapiens (human)] – Gene – NCBI”. www.ncbi.nlm.nih.gov. Retrieved 2019-10-13.
- “PAEP – Glycodelin precursor – Homo sapiens (Human) – PAEP gene & protein”. www.uniprot.org. Retrieved 2019-10-23.
Function
GD is the most important protein secreted in the endometrium during the mid-luteal phase of the menstrual cycle and during the first semester of pregnancy. Four distinct forms of glycoprotein, with identical protein backbones but different glycosylation profiles, are found in amniotic fluid, follicular fluid and seminal plasma of the reproductive system. These glycoproteins have distinct and essential roles in regulating a uterine environment suitable for pregnancy and in the timing and occurrence of the appropriate sequence of events in the fertilization process.
Glycodelin-A
In the female genital tract is mainly expressed in EECs (cultured endometrial epithelial cells) and secreted into the amniotic fluid, endometrium/decidua and maternal serum. Glycodelin-A has contraceptive and immunosuppressive functions, due to the fact that suppresses Natural Killer cells, achieving the prevention of the maternal rejection of the fetus at the fetomaternal interface. It has a molecular weight of 18.78 KDa determined from the cDNA sequence.
- JULKUNEN, MERVI; KOISTINEN, RIITTA; SJÖBERG, JARI; RUTANEN, EEVA-MARJA; WAHLSTRÖM, TORSTEN; SEPPÄLÄ, MARKKU (May 1986). “Secretory Endometrium Synthesizes Placental Protein 14*”. Endocrinology. 118 (5): 1782–1786. doi:10.1210/endo-118-5-1782. ISSN 0013-7227. PMID 3516653.
- Kao, L. C.; Tulac, S.; Lobo, S.; Imani, B.; Yang, J. P.; Germeyer, A.; Osteen, K.; Taylor, R. N.; Lessey, B. A.; Giudice, L. C. (June 2002). “Global Gene Profiling in Human Endometrium during the Window of Implantation”. Endocrinology. 143 (6): 2119–2138. doi:10.1210/endo.143.6.8885. ISSN 0013-7227. PMID 12021176.
- Alok, Anshula; Mukhopadhyay, Debaditya; Karande, Anjali A. (May 2009). “Glycodelin A, an immunomodulatory protein in the endometrium, inhibits proliferation and induces apoptosis in monocytic cells”. The International Journal of Biochemistry & Cell Biology. 41 (5): 1138–1147. doi:10.1016/j.biocel.2008.10.009. ISSN 1357-2725. PMID 18996219.
Glycodelin-S
Is secreted from seminal vesicles to the seminal fluid. A number of alternatively spliced transcript variants have been observed at this locus, but the full-length nature of only two, each encoding the same protein, has been determined. During the passage of the sperm through the cervix, glycodelin S is de-glycosylated and dissociates from the sperm, allowing the sperm to mature.
- Mandelin, Erik; Koistinen, Hannu; Koistinen, Riitta; Arola, Johanna; Affandi, Biran; Seppälä, Markku (September 2001). “Endometrial expression of glycodelin in women with levonorgestrel-releasing subdermal implants”. Fertility and Sterility. 76 (3): 474–478. doi:10.1016/s0015-0282(01)01969-0. ISSN 0015-0282. PMID 11532467.
- Sjöberg, J.; Wahlström, T.; Seppälä, M.; Rutanen, E.-M.; Koistinen, R.; Koskimies, A. I.; Sinosich, M. J.; Teisner, B.; Grudzinskas, J. G. (January 1985). “Seminal Plasma Levels of PAPP-A in Normospermic and Oligospermic Men and Tissue Localization of PAPP-A in the Male Genital Tract”. Archives of Andrology. 14 (2–3): 253–261. doi:10.3109/01485018508988308. ISSN 0148-5016. PMID 2415076.
- Koistinen, Hannu; Koistinen, Riitta; Dell, Anne; Morris, Howard R.; Easton, Richard L.; Patankar, Manish S.; Oehninger, Sergio; Clark, Gary F.; Seppälä, Markku (1996). “Glycodelin from seminal plasma is a differentially glycosylated form of contraceptive glycodelin-A”. Molecular Human Reproduction. 2 (10): 759–765. doi:10.1093/molehr/2.10.759. ISSN 1360-9947. PMID 9239694.
Glycodelin-F
Is secreted by granulosa cells into the follicular fluid. Glycodelin-F reduces the blinding of spermatozoa to the zona pellucida which is mainly expressed in the ovary, and synthesised in the granulosa cells, has a function in principle similar to that of Glycodelin-A. It also binds the sperm head, thereby inhibiting acrosome reaction and sperm-egg binding. Upon de-glycosilation, glycodelin F dissociates from the sperm and sperm-egg binding is possible. The de-glycosilation takes place during the passage of the sperm through the corona cell layer. Glycodelin F is thereby important to prevent a premature acrosome reaction.
- Chiu, Philip C. N.; Chung, Man-Kin; Koistinen, Riitta; Koistinen, Hannu; Seppala, Markku; Ho, Pak-Chung; Ng, Ernest H. Y.; Lee, Kai-Fai; Yeung, William S. B. (2006-12-27). “Cumulus Oophorus-associated Glycodelin-C Displaces Sperm-bound Glycodelin-A and -F and Stimulates Spermatozoa-Zona Pellucida Binding”. Journal of Biological Chemistry. 282 (8): 5378–5388. doi:10.1074/jbc.m607482200. ISSN 0021-9258. PMID 17192260.
- Kölbl, Alexandra C.; Andergassen, Ulrich; Jeschke, Udo (2015-10-13). “The Role of Glycosylation in Breast Cancer Metastasis and Cancer Control”. Frontiers in Oncology. 5: 219. doi:10.3389/fonc.2015.00219. ISSN 2234-943X. PMC 4602128. PMID 26528431.
Glycodelin-C
Found in Cumulus Oophorus, stimulates binding of the spermatozoa to the zona pellucida. First, cumulus cells reduce the spermatozoa-zona binding inhibitory activity of follicular fluid probably by taking up and converting glycodelin-A and glycodelin-F into glycodelin-C. Second, spermatozoa have enhanced zona binding ability after penetrating through the cumulus oophorus. (Cumulus oophorus means “egg-bearing little cloud.” As a sperm enters the cumulus oophorus, the enzyme hyaluronidase on the sperm head dissolves hyaluronic acid, a major component of the cementing material found between the cells of the cumulus oophorus as well as between other cells in the body.) Glycodelin-C is responsible for the latter observation.
- Chiu, Philip C. N.; Chung, Man-Kin; Koistinen, Riitta; Koistinen, Hannu; Seppala, Markku; Ho, Pak-Chung; Ng, Ernest H. Y.; Lee, Kai-Fai; Yeung, William S. B. (2006-12-27). “Cumulus Oophorus-associated Glycodelin-C Displaces Sperm-bound Glycodelin-A and -F and Stimulates Spermatozoa-Zona Pellucida Binding”. Journal of Biological Chemistry. 282 (8): 5378–5388. doi:10.1074/jbc.m607482200. ISSN 0021-9258. PMID 17192260.
- Yeung, William S.B.; Lee, Kai-Fai; Koistinen, Riitta; Koistinen, Hannu; Seppälä, Markku; Chiu, Philip C.N. (December 2009). “Effects of glycodelins on functional competence of spermatozoa”. Journal of Reproductive Immunology. 83 (1–2): 26–30. doi:10.1016/j.jri.2009.04.012. ISSN 0165-0378. PMID 19857900.
- Cumulus Oophorus at ScienceDirect https://www.sciencedirect.com/topics/medicine-and-dentistry/cumulus-oophorus
| GLYCOFORM | SOURCE | GLYCOSYLATION | REPRODUCTIVE FUNCTIONS |
|---|---|---|---|
| GdA | Amniotic fluid, pregnancy decidua | High sialylation, more fucosylation | Immunoprotection for implantation and placentation, antifertilizing, inhibiting spermatozoa-zona pellucida binding |
| GdS | Seminal plasma, seminal vesicles | No sialylated glycans, rich in fucose and mannose | Preventing premature capacitation |
| GdF | Ovarian follicles, oviduct | Fucosylated Lewis-x and Lewis-y, more N-acetylglucosamine | Inhibiting spermatozoa-zona pellucida, preventing premature acrosome reaction |
| GdC | Cumulus oophorus, converted from GdA and GdF | Reacting with specific agglutinins in lectin-binding manner | Stimulating spermatozoa-zona pellucida binding |
- Cui, Juan; Liu, Yanguo; Wang, Xiuwen (2017-11-29). “The Roles of Glycodelin in Cancer Development and Progression”. Frontiers in Immunology. 8: 1685. doi:10.3389/fimmu.2017.01685. ISSN 1664-3224. PMC 5712544. PMID 29238349.
Sialylation refers to the terminal addition of sialic acid units to oligosaccharides and glycoproteins. Sialylation of glycoproteins and glycopilids finds role in embryonic development, neurodevelopment, reprogramming, oncogenesis, and immune responses. It has recently reported of role in cell fate decision during development, reprogramming, and cancer progression.
- Sialyation at ScienceDirect
- Li, F., Ding, J. Sialylation is involved in cell fate decision during development, reprogramming and cancer progression. Protein Cell 10, 550–565 (2019). https://doi.org/10.1007/s13238-018-0597-5
Fucosylation is the process of transferring fucose from GDP-fucose to their substrates, which includes certain proteins, N- and O-linked glycans in glycoprotein or glycolipids, by fucosyltransferases in all mammalian cells. Fucosylated glycans play vital role in selectin-mediated leukocyte extravasation, lymphocyte homing, and pathogen–host interactions, whereas fucosylated proteins are essential for signaling transduction in numerous ontogenic events. Aberrant fucosylation due to the availability of high energy donor GDP-fucose, abnormal expression of FUTs and/or α-fucosidase, and the availability of their substrates leads to different fucosylated glycan or protein structures. Accumulating evidence demonstrates that aberrant fucosylation plays important role in all aspects of cancer biology.
- Fucosylation at Science Direct
- M Shan, D Yang, H Dou, L Zhang Fucosylation in cancer biology and its clinical applications, Progress in Molecular Biology and Translational Science Volume 162, 2019, Pages 93-119 https://doi.org/10.1016/bs.pmbts.2019.01.002
Fucose is a hexose deoxy sugar with the chemical formula C6H12O5. It is found on N-linked glycans on the mammalian, insect and plant cell surface. Fucose is the fundamental sub-unit of the seaweed polysaccharide fucoidan. The α(1→3) linked core of fucoidan is a suspected carbohydrate antigen for IgE-mediated allergy. Two structural features distinguish fucose from other six-carbon sugars present in mammals: the lack of a hydroxyl group on the carbon at the 6-position (C-6) (thereby making it a deoxy sugar) and the L-configuration. It is equivalent to 6-deoxy-l-galactose. In the fucose-containing glycan structures, fucosylated glycans, fucose can exist as a terminal modification or serve as an attachment point for adding other sugars. In human N-linked glycans, fucose is most commonly linked α-1,6 to the reducing terminal β-N-acetylglucosamine. However, fucose at the non-reducing termini linked α-1,2 to galactose forms the H antigen, the substructure of the A and B blood group antigens.
H antigen (“H substance” redirects here). The term also historically referred to histamine. H antigen can refer to one of various types of antigens having diverse biological functions:
- Also known as substance H, H antigen is a precursor to each of the ABO blood group antigens, apparently present in all people except those with the Bombay Blood phenotype (see hh blood group). The gene responsible for making H antigen is FUT1, located on the 19th chromosome in humans
- Histocompatibility antigen, a major factor in graft rejection. Even when Major Histocompatibility Complex genotype is perfectly matched, can cause slow rejection of a graft.
- major H antigens “encode molecules that present foreign peptides to T cells“
- minor H antigens “present polymorphic self peptides to T cells”. Includes, e.g. the H-Y antigen
- a bacterial flagellar antigen
- Science Of Biogenetics. “Do you Know Bombay Blood Group”.
- Janeway, Charles A. (2001). Immunobiology the immune system health & disease (5. ed.). New York: Garland. ISBN 978-0-8153-3642-6. Retrieved 16 December 2013.
- Farlex. “antigen”. The Free Dictionary. Retrieved 16 December 2013.
Fucose is released from fucose-containing polymers by an enzyme called α-fucosidase found in lysosomes. l-Fucose has several potential applications in cosmetics, pharmaceuticals, and dietary supplements Fucosylation of antibodies has been established to reduce binding to the Fc receptor of Natural Killer cells and thereby reduce antigen-dependent cellular cytotoxicity. Therefore, afucosylated monoclonal antibodies have been designed to recruit the immune system to cancers cells have been manufactured in cell lines deficient in the enzyme for core fucosylation (FUT8), thereby enhancing the in vivo cell killing.
- Alpha-(1,6)-fucosyltransferase is an enzyme that in humans is encoded by the FUT8 gene. This enzyme belongs to the family of fucosyltransferases. The product of this gene catalyzes the transfer of fucose from GDP-fucose to N-linked type complex glycopeptides. This enzyme is distinct from other fucosyltransferases which catalyze alpha1-2, alpha1-3, and alpha1-4 fucose addition. The expression of this gene may contribute to the malignancy of cancer cells and to their invasive and metastatic capabilities. Alternatively spliced variants encoding different isoforms have been identified. Kyowa Hakko Kirin‘s “Potelligent” platform uses a CHO cell line in which FUT8 has been knocked out to make afucosylated monoclonal antibodies.
- Chinese hamster ovary (CHO) cells are an epithelialcell line derived from the ovary of the Chinese hamster, often used in biological and medical research and commercially in the production of recombinant therapeutic proteins. Chinese hamsters had been used in research since 1919, where they were used in place of mice for typing pneumococci. They were subsequently found to be excellent vectors for transmission of kala-azar (visceral leishmaniasis), facilitating Leishmania research. In 1948, the Chinese hamster was first used in the United States for breeding in research laboratories. In 1957, Theodore T. Puck obtained a female Chinese hamster from Dr. George Yerganian’s laboratory at the Boston Cancer Research Foundation and used it to derive the original Chinese hamster ovary (CHO) cell line. Since then, CHO cells have been a cell line of choice because of their rapid growth in suspension culture and high protein production. The thrombolytic medication alteplase (Activase), which was approved by the US Food and Drug Administration in 1987, was the first commercially available recombinant protein produced from CHO cells. CHO cells have played a significant role in the production of recombinant protein therapeutics since, with CHO cells producing 70% of biologics and monoclonal antibodies in 2016. All CHO cell lines are deficient in proline synthesis. Also, CHO cells do not express the epidermal growth factor receptor (EGFR), which makes them ideal in the investigation of various EGFR mutations. Furthermore, Chinese hamster ovary cells are able to produce proteins with complex glycosylations, post-translational modifications (PTMs) similar to those produced in humans. They are easily growable in large-scale cultures and have great viability, which is why they are ideal for GMP protein production. Also, CHO cells are tolerant to variations in parameters, be it oxygen levels, pH-value, temperature or cell density.
- Wurm FM (2004). “Production of recombinant protein therapeutics in cultivated mammalian cells”. Nature Biotechnology. 22 (11): 1393–1398. doi:10.1038/nbt1026. PMID 15529164. S2CID 20428452.
- “Vital Tools A Brief History of CHO Cells” (PDF). LSF Magazine. Winter 2015. pp. 38–47. Retrieved 5 April 2023.
- Young C, Smyly H, Brown C (March 1924). “Experimental kala-azar in a hamster”. Experimental Biology and Medicine. 21 (6): 357–359. doi:10.3181/00379727-21-182. ISSN 1535-3702.
- Fanelli, Alex (2016). “CHO Cells”. Retrieved 28 November 2017.
- Du C; Webb C (2011). “Cellular Systems”. Comprehensive Biotechnology. Elsevier. pp. 11–23. doi:10.1016/b978-0-08-088504-9.00080-5. ISBN 9780080885049.
- Tihanyi B, Nyitray L (December 2020). “Recent advances in CHO cell line development for recombinant protein production”. Drug Discovery Today. 38: 25–34. doi:10.1016/j.ddtec.2021.02.003. hdl:10831/82853. PMID 34895638.
However, 70% of biologics, and almost all mAbs, are produced in Chinese hamster ovary (CHO) cells, as the most commonly used and preferred hosts for biopharmaceutical protein production.
- Liang K, Luo H, Li Q (2023). “Enhancing and stabilizing monoclonal antibody production by Chinese hamster ovary (CHO) cells with optimized perfusion culture strategies”. Frontiers in Bioengineering and Biotechnology. 11: 1112349. doi:10.3389/fbioe.2023.1112349. PMC 9895834. PMID 36741761.
Since 2016, about 70% of all rBPs and mAbs were produced from Chinese hamster ovary (CHO) cell lines
- Wurm FM; Hacker D (2011). “First CHO genome”. Nature Biotechnology. 29 (8): 718–20. doi:10.1038/nbt.1943. PMID 21822249. S2CID 8422581.
- Ahsan, A.; S. M. Hiniker; M. A. Davis; T. S. Lawrence; M. K. Nyati (2009). “Role of Cell Cycle in Epidermal Growth Factor Receptor Inhibitor-Mediated Radiosensitization”. Cancer Research. 69 (12): 5108–5114. doi:10.1158/0008-5472.CAN-09-0466. PMC 2697971. PMID 19509222.
- “CHO cells – 7 facts about the cell line derived from the ovary of the Chinese hamster”. evitria AG. 3 May 2022.
- Tjio J. H.; Puck T. T. (1958). “Genetics of somatic mammalian cells. II. chromosomal constitution of cells in tissue culture”. J. Exp. Med. 108 (2): 259–271. doi:10.1084/jem.108.2.259. PMC 2136870. PMID 13563760.
- Theodore Thomas Puck (September 24, 1916 – November 6, 2005) was an American geneticist born in Chicago, Illinois. He attended Chicago public schools and obtained his bachelors, masters, and doctoral degree from the University of Chicago. His PhD work was on the laws governing the impact of an electron upon an atom and his doctoral adviser was James Franck (After the Nazi Party came to power in Germany in 1933, Franck resigned his post in protest against the dismissal of fellow academics. He assisted Frederick Lindemann in helping dismissed Jewish scientists find work overseas, before he left Germany in November 1933. Franck participated in the Manhattan Project during World War II as Director of the Chemistry Division of the Metallurgical Laboratory. He was also the chairman of the Committee on Political and Social Problems regarding the atomic bomb, which is best known for the compilation of the Franck Report). During WW II Puck stayed at the University of Chicago. There he worked in the laboratory of Oswald H. Robertson (English-born medical scientist who pioneered the idea of blood banks in the “blood depots” he established in 1917 during service in France with the US Army Medical Corps.)on the study of how bacteria and viruses can spread through the air and on dust particles. After a postdoc position in the laboratory of Renato Dulbecco (Italian–American virologist who won the 1975 Nobel Prize in Physiology or Medicine for his work on oncoviruses, which are viruses that can cause cancer when they infect animal cells), Puck was recruited in 1948 to establish and chair the University of Colorado Medical School‘s department of biophysics. He retired from the University of Colorado Medical School in 1995 as professor emeritus, but continued to do laboratory work there until a few weeks before his death. Puck was an early pioneer of “somatic cell genetics” and single-cell plating ( i.e. “cloning” .) This work allowed the genetics of human and other mammalian cells to be studied in detail. Puck’s key work ultimately made modern genetics, such as the human genome and other mammalian genome projects, possible. Dr. Puck with the assistance of Philip I. Marcus, successfully cloned a HeLa cell in 1955. Puck made many basic discoveries in several areas. Confirming research done in 1956 by Joe Hin Tjio, Puck’s team found that humans had 46 chromosomes rather than 48 which had earlier been believed. He developed the CHO cell line from Chinese hamster ovarian cells for this work. These cells are still widely utilized in the bio pharmaceutical industry. Puck studied X-rays and cellular mutations. He also isolated and studied cellular mutations.
- Pincock, Stephen (10 December 2005). “Obituary. Theodore Puck”. The Lancet. 366: 2000. doi:10.1016/S0140-6736(05)67806-3. S2CID 54375562.
- Tjio, J. H.; Puck, Theodore T. (1 August 1958). “Genetics of somatic mammalian cells. II. Chromosomal constitution of cells in tissue culture”. Journal of Experimental Medicine. 108 (2): 259–268. doi:10.1084/jem.108.2.259. PMC 2136870. PMID 13563760.
- Puck, Theodore T.; Marcus, Philip I. (1 May 1956). “Action of X-rays on mammalian cells”. Journal of Experimental Medicine. 103 (5): 653–666. doi:10.1084/jem.103.5.653. PMC 2136626. PMID 13319584.
- Puck’s page at the Eleanor Roosevelt Institute
- “Theodore T. Puck.” Notable Scientists: From 1900 to the Present Gale Group, 2001 Reproduced in Biography Resource Center. Farmington Hills, Mich.: Thomson Gale. 2005
- Science Daily obituary November 11, 2005
- Rocky Mountain News, obituary November 9, 2005[permanent dead link]
- Tribute by Dr. Gordon Sato & colleagues, In Vitro Cell. Devel. Biol-Animal, 2006 Archived 2010-12-05 at the Wayback Machine
- Theodore Thomas Puck (September 24, 1916 – November 6, 2005) was an American geneticist born in Chicago, Illinois. He attended Chicago public schools and obtained his bachelors, masters, and doctoral degree from the University of Chicago. His PhD work was on the laws governing the impact of an electron upon an atom and his doctoral adviser was James Franck (After the Nazi Party came to power in Germany in 1933, Franck resigned his post in protest against the dismissal of fellow academics. He assisted Frederick Lindemann in helping dismissed Jewish scientists find work overseas, before he left Germany in November 1933. Franck participated in the Manhattan Project during World War II as Director of the Chemistry Division of the Metallurgical Laboratory. He was also the chairman of the Committee on Political and Social Problems regarding the atomic bomb, which is best known for the compilation of the Franck Report). During WW II Puck stayed at the University of Chicago. There he worked in the laboratory of Oswald H. Robertson (English-born medical scientist who pioneered the idea of blood banks in the “blood depots” he established in 1917 during service in France with the US Army Medical Corps.)on the study of how bacteria and viruses can spread through the air and on dust particles. After a postdoc position in the laboratory of Renato Dulbecco (Italian–American virologist who won the 1975 Nobel Prize in Physiology or Medicine for his work on oncoviruses, which are viruses that can cause cancer when they infect animal cells), Puck was recruited in 1948 to establish and chair the University of Colorado Medical School‘s department of biophysics. He retired from the University of Colorado Medical School in 1995 as professor emeritus, but continued to do laboratory work there until a few weeks before his death. Puck was an early pioneer of “somatic cell genetics” and single-cell plating ( i.e. “cloning” .) This work allowed the genetics of human and other mammalian cells to be studied in detail. Puck’s key work ultimately made modern genetics, such as the human genome and other mammalian genome projects, possible. Dr. Puck with the assistance of Philip I. Marcus, successfully cloned a HeLa cell in 1955. Puck made many basic discoveries in several areas. Confirming research done in 1956 by Joe Hin Tjio, Puck’s team found that humans had 46 chromosomes rather than 48 which had earlier been believed. He developed the CHO cell line from Chinese hamster ovarian cells for this work. These cells are still widely utilized in the bio pharmaceutical industry. Puck studied X-rays and cellular mutations. He also isolated and studied cellular mutations.
- Chinese hamster ovary (CHO) cells are an epithelialcell line derived from the ovary of the Chinese hamster, often used in biological and medical research and commercially in the production of recombinant therapeutic proteins. Chinese hamsters had been used in research since 1919, where they were used in place of mice for typing pneumococci. They were subsequently found to be excellent vectors for transmission of kala-azar (visceral leishmaniasis), facilitating Leishmania research. In 1948, the Chinese hamster was first used in the United States for breeding in research laboratories. In 1957, Theodore T. Puck obtained a female Chinese hamster from Dr. George Yerganian’s laboratory at the Boston Cancer Research Foundation and used it to derive the original Chinese hamster ovary (CHO) cell line. Since then, CHO cells have been a cell line of choice because of their rapid growth in suspension culture and high protein production. The thrombolytic medication alteplase (Activase), which was approved by the US Food and Drug Administration in 1987, was the first commercially available recombinant protein produced from CHO cells. CHO cells have played a significant role in the production of recombinant protein therapeutics since, with CHO cells producing 70% of biologics and monoclonal antibodies in 2016. All CHO cell lines are deficient in proline synthesis. Also, CHO cells do not express the epidermal growth factor receptor (EGFR), which makes them ideal in the investigation of various EGFR mutations. Furthermore, Chinese hamster ovary cells are able to produce proteins with complex glycosylations, post-translational modifications (PTMs) similar to those produced in humans. They are easily growable in large-scale cultures and have great viability, which is why they are ideal for GMP protein production. Also, CHO cells are tolerant to variations in parameters, be it oxygen levels, pH-value, temperature or cell density.
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. (2017-09-01). “Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification”. Food Research International. 99 (Pt 3): 1011–1020. doi:10.1016/j.foodres.2016.11.016. hdl:10197/8191. ISSN 0963-9969. PMID 28865611. S2CID 10531419.
- Daniel J. Becker; John B. Lowe (July 2003). “Fucose: biosynthesis and biological function in mammals”. Glycobiology. 13 (7): 41R–53R. doi:10.1093/glycob/cwg054. PMID 12651883.
- Daniel J. Moloney; Robert S. Haltiwanger (July 1999). “The O-linked fucose glycosylation pathway: identification and characterization of a uridine diphosphoglucose: fucose-[beta]1,3-glucosyltransferase activity from Chinese hamster ovary cells”. Glycobiology. 9 (7): 679–687. doi:10.1093/glycob/9.7.679. PMID 10362837.
- Roca, C (2015). “Exopolysaccharides enriched in rare sugars: bacterial sources, production, and applications”. Front Microbiol. 6: 288. doi:10.3389/fmicb.2015.00288. PMC 4392319. PMID 25914689.
- Vanhooren, PT (1999). “L-fucose: occurrence, physiological role, chemical, enzymatic and microbial synthesis”. J. Chem. Technol. Biotechnol. 74 (6): 479–497. doi:10.1002/(SICI)1097-4660(199906)74:6<479::AID-JCTB76>3.0.CO;2-E.
- Dalziel, Martin; Crispin, Max; Scanlan, Christopher N.; Zitzmann, Nicole; Dwek, Raymond A. (2014-01-03). “Emerging Principles for the Therapeutic Exploitation of Glycosylation”. Science. 343 (6166): 1235681. doi:10.1126/science.1235681. ISSN 0036-8075. PMID 24385630. S2CID 206548002.
- Yu, X; Marshall, MJE; Cragg, MS; Crispin, M (June 2017). “Improving Antibody-Based Cancer Therapeutics Through Glycan Engineering” (PDF). BioDrugs. 31 (3): 151–166. doi:10.1007/s40259-017-0223-8. PMID 28466278. S2CID 3722081.
Level concentrations
PP-14 is found in the oocyte and in the sperm. In men, the concentration of this protein in seminal plasma is higher than those in serum. In women, the levels in follicular fluid exceed those of non-pregnant women.
- SEPPäLä, MARKKU; KOSKIMIES, AARNE I.; TENHUNEN, ANSSI; RUTANEN, EEVA-MARJA; SJÖBERG, JARI; KOISTINEN, RIITTA; JULKUNEN, MERVI; WAHLSTRÖM, TORSTEN (May 1985). “Pregnancy Proteins in Seminal Plasma, Seminal Vesicles, Preovulatory Follicular Fluid, and Ovary”. Annals of the New York Academy of Sciences. 442 (1 In Vitro Fert): 212–226. Bibcode:1985NYASA.442..212S. doi:10.1111/j.1749-6632.1985.tb37522.x. ISSN 0077-8923. PMID 3893267. S2CID 11729995.
·Seminal plasma:
PP-14 is a significant protein constituent in most seminal plasma samples of men; sometimes comprising over 2.5% of the total protein content. The concentration of PP-14 in seminal plasma from men with oligospermia is in the reference range of this protein derived from values measured in normal men. However, vasectomized men concentrations are less than normal.
- Bolton, A. E.; Pinto-Furtado, L. G.; Andrew, C. E.; Chapman, M. G. (June 1986). “Measurement of the pregnancy-associated proteins, placental protein 14 and pregnancy-associated plasma protein A in human seminal plasma”. Clinical Reproduction and Fertility. 4 (3): 233–240. ISSN 0725-556X. PMID 2427179.
·Women’s tissues and body fluids:
In serum of non-pregnant women, the concentration of PP-14 is approximately 15-40 µg/L.
In normal pregnancy:
| Location (tissues and body fluids) | PP-14 concentrations (approximately) | Time |
| Serum | Up to 2200 µg/L (highest) | 6–12 weeks |
| — | Decreasing concentrations | After 16 weeks |
| — | 200 µg/L approx. | 24 weeks (plateaued) |
| Amniotic fluid | 232 mg/L (highest) (higher than those in maternal serum throughout pregnancy) | 12–20 weeks |
| Cord blood | 15-22 µg or undetectable | ———— |
| Early pregnancy decidua | 41–160 mg/g total protein | ———— |
| Late pregnancy decidua | 60-2700 µg/g total protein | ———— |
| Amnion and chorion laeve | From 50 to 750 µg/g protein | ———— |
| From 50 to 1000 µg/g protein | ———— | |
| Early pregnancy placenta | 0.25–15 mg/g | ———— |
| Late pregnancy placenta | 3-430 µg/g protein | ———— |
The concentrations of PP-14 in pregnancy serum are comparable with hCG (Human Chorionic Gonadotropin). Among all the placental proteins, the amniotic fluid PP-14 concentration is the most outstanding as decidua is a source of this protein.
- JULKUNEN, MERVI; RUTANEN, EEVA-MARJA; KOSKIMIES, AARNE; RANTA, TAPIO; BOHN, HANS; SEPPALA, MARKKU (November 1985). “Distribution of placental protein 14 in tissues and body fluids during pregnancy”. BJOG: An International Journal of Obstetrics and Gynaecology. 92 (11): 1145–1151. doi:10.1111/j.1471-0528.1985.tb03027.x. ISSN 1470-0328. PMID 4063232. S2CID 40266453.
Future clinical applications
Placental Protein 14 has some clinical applications:
1. Biomarker of premature rupture of membranes
Premature rupture of membranes is a common pregnancy complication, taking into account that the current method does not satisfy the medical community, some researches have determined a new method: the analysis of placental protein in the maternal plasma and vaginal fluid. The results of these studies have shown that PP-14’s concentration increased in the case of premature rupture of membranes. So this study conclude that PP-14 is an excellent biomarker with a sensibility of 100% and a specificity of 87,5%.
- Yanyun , Haibo, Guanglu, Yanqin, Jun, Qiongli,Qiongli, Linbo ,Tao, Wang, Luo, Che, Li, Gao,Yang, Zhou, Gao, Wang. (2018). “Placental protein 14 as a potential biomarker for diagnosis of preterm premature rupture of membranes”. Molecular Medicine Reports. 18 (1): 113–122. doi:10.3892/mmr.2018.8967. PMC 6059659. PMID 29749501. Retrieved 25 October 2019.
2. Biomarker in in vitro fertilization process
PP-14 is known to be a great marker to predict the outcome of in vitro fertilization and the embryo transfer cycle. Some studies have shown that the serum concentration of Placental Protein 14 was highly increased after the embryo transfer cycle, and they conclude that PP-14 might be an excellent marker to predict the endometrial receptivity.
- Suzuki, Fukumine, Sugiyama, Usuda, Yoshichika, Noritaka, Rikikazu, Saburo. (2000). “Clinical Applications of Serum Placental Protein 14 (PP14) Measurement in the IVF-ET Cycle”. Journal of Obstetrics and Gynaecology Research. 26 (4): 295–302. doi:10.1111/j.1447-0756.2000.tb01325.x. PMID 11049241. S2CID 22904564. Retrieved 25 October 2019.
References
- GRCh38: Ensembl release 89: ENSG00000122133 – Ensembl, May 2017
- “Human PubMed Reference:”. National Center for Biotechnology Information, U.S. National Library of Medicine.
- “PAEP – Glycodelin precursor – Homo sapiens (Human) – PAEP gene & protein”. www.uniprot.org.
- Wang, Ping; Libho, Zhu; Xinmei, Zhang (December 2013). “The role of Placental Protein 14 in the Pathogenesis of Endometrosis”. Reproductive Sciences. 20 (12): 1465–1470. doi:10.1177/1933719113488452. ISSN 0077-8923. PMC 3817670. PMID 23670949.
- SEPPÄLÄ, MARKKU; JULKUNEN, MERVI; KOSKIMIES, AARNE; LAATIKAINEN, TIMO; STENMAN, ULF–HÅKAN; HUHTALA, MARJA-LIISA (October 1988). “Proteins of the Human Endometrium”. Annals of the New York Academy of Sciences. 541 (1): 432–444. Bibcode:1988NYASA.541..432S. doi:10.1111/j.1749-6632.1988.tb22280.x. ISSN 0077-8923. PMID 3195927. S2CID 222073546.
- Dutta, Binita; Mukhopadhyay, Debaditya; Roy, Nita; Das, Goutam; Karande, Anjali A. (December 1998). “Cloning, Expression, Purification, and Immunocharacterization of Placental Protein-14”. Protein Expression and Purification. 14 (3): 327–334. doi:10.1006/prep.1998.0961. ISSN 1046-5928. PMID 9882566.
- Julkunen, M.; Seppala, M.; Janne, O. A. (1988-12-01). “Complete amino acid sequence of human placental protein 14: a progesterone-regulated uterine protein homologous to beta-lactoglobulins”. Proceedings of the National Academy of Sciences. 85 (23): 8845–8849. Bibcode:1988PNAS…85.8845J. doi:10.1073/pnas.85.23.8845. ISSN 0027-8424. PMC 282603. PMID 3194393.
- Julkunen, M.; Seppala, M.; Janne, O. A. (1988-12-01). “Complete amino acid sequence of human placental protein 14: a progesterone-regulated uterine protein homologous to beta-lactoglobulins”. Proceedings of the National Academy of Sciences. 85 (23): 8845–8849. Bibcode:1988PNAS…85.8845J. doi:10.1073/pnas.85.23.8845. ISSN 0027-8424. PMC 282603. PMID 3194393.
- “PAEP progestagen associated endometrial protein [Homo sapiens (human)] – Gene – NCBI”. www.ncbi.nlm.nih.gov. Retrieved 2019-10-13.
- “PAEP – Glycodelin precursor – Homo sapiens (Human) – PAEP gene & protein”. www.uniprot.org. Retrieved 2019-10-23.
- JULKUNEN, MERVI; KOISTINEN, RIITTA; SJÖBERG, JARI; RUTANEN, EEVA-MARJA; WAHLSTRÖM, TORSTEN; SEPPÄLÄ, MARKKU (May 1986). “Secretory Endometrium Synthesizes Placental Protein 14*”. Endocrinology. 118 (5): 1782–1786. doi:10.1210/endo-118-5-1782. ISSN 0013-7227. PMID 3516653.
- Kao, L. C.; Tulac, S.; Lobo, S.; Imani, B.; Yang, J. P.; Germeyer, A.; Osteen, K.; Taylor, R. N.; Lessey, B. A.; Giudice, L. C. (June 2002). “Global Gene Profiling in Human Endometrium during the Window of Implantation”. Endocrinology. 143 (6): 2119–2138. doi:10.1210/endo.143.6.8885. ISSN 0013-7227. PMID 12021176.
- Alok, Anshula; Mukhopadhyay, Debaditya; Karande, Anjali A. (May 2009). “Glycodelin A, an immunomodulatory protein in the endometrium, inhibits proliferation and induces apoptosis in monocytic cells”. The International Journal of Biochemistry & Cell Biology. 41 (5): 1138–1147. doi:10.1016/j.biocel.2008.10.009. ISSN 1357-2725. PMID 18996219.
- Mandelin, Erik; Koistinen, Hannu; Koistinen, Riitta; Arola, Johanna; Affandi, Biran; Seppälä, Markku (September 2001). “Endometrial expression of glycodelin in women with levonorgestrel-releasing subdermal implants”. Fertility and Sterility. 76 (3): 474–478. doi:10.1016/s0015-0282(01)01969-0. ISSN 0015-0282. PMID 11532467.
- Sjöberg, J.; Wahlström, T.; Seppälä, M.; Rutanen, E.-M.; Koistinen, R.; Koskimies, A. I.; Sinosich, M. J.; Teisner, B.; Grudzinskas, J. G. (January 1985). “Seminal Plasma Levels of PAPP-A in Normospermic and Oligospermic Men and Tissue Localization of PAPP-A in the Male Genital Tract”. Archives of Andrology. 14 (2–3): 253–261. doi:10.3109/01485018508988308. ISSN 0148-5016. PMID 2415076.
- Koistinen, Hannu; Koistinen, Riitta; Dell, Anne; Morris, Howard R.; Easton, Richard L.; Patankar, Manish S.; Oehninger, Sergio; Clark, Gary F.; Seppälä, Markku (1996). “Glycodelin from seminal plasma is a differentially glycosylated form of contraceptive glycodelin-A”. Molecular Human Reproduction. 2 (10): 759–765. doi:10.1093/molehr/2.10.759. ISSN 1360-9947. PMID 9239694.
- Chiu, Philip C. N.; Chung, Man-Kin; Koistinen, Riitta; Koistinen, Hannu; Seppala, Markku; Ho, Pak-Chung; Ng, Ernest H. Y.; Lee, Kai-Fai; Yeung, William S. B. (2006-12-27). “Cumulus Oophorus-associated Glycodelin-C Displaces Sperm-bound Glycodelin-A and -F and Stimulates Spermatozoa-Zona Pellucida Binding”. Journal of Biological Chemistry. 282 (8): 5378–5388. doi:10.1074/jbc.m607482200. ISSN 0021-9258. PMID 17192260.
- Kölbl, Alexandra C.; Andergassen, Ulrich; Jeschke, Udo (2015-10-13). “The Role of Glycosylation in Breast Cancer Metastasis and Cancer Control”. Frontiers in Oncology. 5: 219. doi:10.3389/fonc.2015.00219. ISSN 2234-943X. PMC 4602128. PMID 26528431.
- Chiu, Philip C. N.; Chung, Man-Kin; Koistinen, Riitta; Koistinen, Hannu; Seppala, Markku; Ho, Pak-Chung; Ng, Ernest H. Y.; Lee, Kai-Fai; Yeung, William S. B. (2006-12-27). “Cumulus Oophorus-associated Glycodelin-C Displaces Sperm-bound Glycodelin-A and -F and Stimulates Spermatozoa-Zona Pellucida Binding”. Journal of Biological Chemistry. 282 (8): 5378–5388. doi:10.1074/jbc.m607482200. ISSN 0021-9258. PMID 17192260.
- Yeung, William S.B.; Lee, Kai-Fai; Koistinen, Riitta; Koistinen, Hannu; Seppälä, Markku; Chiu, Philip C.N. (December 2009). “Effects of glycodelins on functional competence of spermatozoa”. Journal of Reproductive Immunology. 83 (1–2): 26–30. doi:10.1016/j.jri.2009.04.012. ISSN 0165-0378. PMID 19857900.
- Cui, Juan; Liu, Yanguo; Wang, Xiuwen (2017-11-29). “The Roles of Glycodelin in Cancer Development and Progression”. Frontiers in Immunology. 8: 1685. doi:10.3389/fimmu.2017.01685. ISSN 1664-3224. PMC 5712544. PMID 29238349.
- SEPPäLä, MARKKU; KOSKIMIES, AARNE I.; TENHUNEN, ANSSI; RUTANEN, EEVA-MARJA; SJÖBERG, JARI; KOISTINEN, RIITTA; JULKUNEN, MERVI; WAHLSTRÖM, TORSTEN (May 1985). “Pregnancy Proteins in Seminal Plasma, Seminal Vesicles, Preovulatory Follicular Fluid, and Ovary”. Annals of the New York Academy of Sciences. 442 (1 In Vitro Fert): 212–226. Bibcode:1985NYASA.442..212S. doi:10.1111/j.1749-6632.1985.tb37522.x. ISSN 0077-8923. PMID 3893267. S2CID 11729995.
- Bolton, A. E.; Pinto-Furtado, L. G.; Andrew, C. E.; Chapman, M. G. (June 1986). “Measurement of the pregnancy-associated proteins, placental protein 14 and pregnancy-associated plasma protein A in human seminal plasma”. Clinical Reproduction and Fertility. 4 (3): 233–240. ISSN 0725-556X. PMID 2427179.
- JULKUNEN, MERVI; RUTANEN, EEVA-MARJA; KOSKIMIES, AARNE; RANTA, TAPIO; BOHN, HANS; SEPPALA, MARKKU (November 1985). “Distribution of placental protein 14 in tissues and body fluids during pregnancy”. BJOG: An International Journal of Obstetrics and Gynaecology. 92 (11): 1145–1151. doi:10.1111/j.1471-0528.1985.tb03027.x. ISSN 1470-0328. PMID 4063232. S2CID 40266453.
- Yanyun , Haibo, Guanglu, Yanqin, Jun, Qiongli,Qiongli, Linbo ,Tao, Wang, Luo, Che, Li, Gao,Yang, Zhou, Gao, Wang. (2018). “Placental protein 14 as a potential biomarker for diagnosis of preterm premature rupture of membranes”. Molecular Medicine Reports. 18 (1): 113–122. doi:10.3892/mmr.2018.8967. PMC 6059659. PMID 29749501. Retrieved 25 October 2019.
- Suzuki, Fukumine, Sugiyama, Usuda, Yoshichika, Noritaka, Rikikazu, Saburo. (2000). “Clinical Applications of Serum Placental Protein 14 (PP14) Measurement in the IVF-ET Cycle”. Journal of Obstetrics and Gynaecology Research. 26 (4): 295–302. doi:10.1111/j.1447-0756.2000.tb01325.x. PMID 11049241. S2CID 22904564. Retrieved 25 October 2019.
Further reading
- Seppälä M, Bohn H, Tatarinov Y (1998). “Glycodelins”. Tumour Biology. 19 (3): 213–20. doi:10.1159/000030009. PMID 9591048. S2CID 232276875.
- Salier JP (Oct 2000). “Chromosomal location, exon/intron organization and evolution of lipocalin genes”. Biochimica et Biophysica Acta (BBA) – Protein Structure and Molecular Enzymology. 1482 (1–2): 25–34. doi:10.1016/S0167-4838(00)00144-8. PMID 11058744.
- Halttunen M, Kämäräinen M, Koistinen H (Oct 2000). “Glycodelin: a reproduction-related lipocalin”. Biochimica et Biophysica Acta (BBA) – Protein Structure and Molecular Enzymology. 1482 (1–2): 149–56. doi:10.1016/S0167-4838(00)00158-8. PMID 11058757.
- Seppälä M, Taylor RN, Koistinen H, Koistinen R, Milgrom E (Aug 2002). “Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation”. Endocrine Reviews. 23 (4): 401–30. doi:10.1210/er.2001-0026. PMID 12202458.
- Seppälä M, Koistinen H, Koistinen R, Chiu PC, Yeung WS (2007). “Glycosylation related actions of glycodelin: gamete, cumulus cell, immune cell and clinical associations”. Human Reproduction Update. 13 (3): 275–87. doi:10.1093/humupd/dmm004. PMID 17329396.
- Garde J, Bell SC, Eperon IC (Mar 1991). “Multiple forms of mRNA encoding human pregnancy-associated endometrial alpha 2-globulin, a beta-lactoglobulin homologue”. Proceedings of the National Academy of Sciences of the United States of America. 88 (6): 2456–60. Bibcode:1991PNAS…88.2456G. doi:10.1073/pnas.88.6.2456. PMC 51251. PMID 2006183.
- Wood PL, Iffland CA, Allen E, Bentick B, Burton P, Shaw RW, Bell SC (May 1990). “Serum levels of pregnancy-associated endometrial alpha 2-globulin (alpha 2-PEG), a glycosylated beta-lactoglobulin homologue, in successful and unsuccessful assisted conception”. Human Reproduction. 5 (4): 421–6. doi:10.1093/oxfordjournals.humrep.a137115. PMID 2113930.
- Vaisse C, Atger M, Potier B, Milgrom E (1990). “Human placental protein 14 gene: sequence and characterization of a short duplication”. DNA and Cell Biology. 9 (6): 401–13. doi:10.1089/dna.1990.9.401. PMID 2206398.
- Check JH, Nowroozi K, Chase JS, Vaze M, Joshi S, Baker AF (Jun 1990). “Serum progestagen-associated endometrial protein (PEP) levels in conception versus nonconception cycles following in vitro fertilization-embryo transfer”. Journal of in Vitro Fertilization and Embryo Transfer. 7 (3): 134–6. doi:10.1007/BF01135675. PMID 2380618. S2CID 12383913.
- Wood PL, Waites GT, MacVicar J, Davidson AC, Walker RA, Bell SC (Dec 1988). “Immunohistological localization of pregnancy-associated endometrial alpha 2-globulin (alpha 2-PEG) in endometrial adenocarcinoma and effect of medroxyprogesterone acetate”. British Journal of Obstetrics and Gynaecology. 95 (12): 1292–8. doi:10.1111/j.1471-0528.1988.tb06820.x. PMID 2975952. S2CID 71096539.
- Joshi SG (1987). “Progestin-Dependent Human Endometrial Protein: A Marker for Monitoring Human Endometrial Function”. Cell and Molecular Biology of the Uterus. Advances in Experimental Medicine and Biology. Vol. 230. pp. 167–86. doi:10.1007/978-1-4684-1297-0_10. ISBN 978-1-4684-1299-4. PMID 3135704.
- Julkunen M, Seppälä M, Jänne OA (Dec 1988). “Complete amino acid sequence of human placental protein 14: a progesterone-regulated uterine protein homologous to beta-lactoglobulins”. Proceedings of the National Academy of Sciences of the United States of America. 85 (23): 8845–9. Bibcode:1988PNAS…85.8845J. doi:10.1073/pnas.85.23.8845. PMC 282603. PMID 3194393.
- Huhtala ML, Seppälä M, Närvänen A, Palomäki P, Julkunen M, Bohn H (Jun 1987). “Amino acid sequence homology between human placental protein 14 and beta-lactoglobulins from various species”. Endocrinology. 120 (6): 2620–2. doi:10.1210/endo-120-6-2620. PMID 3569148.
- Bell SC, Keyte JW, Waites GT (Nov 1987). “Pregnancy-associated endometrial alpha 2-globulin, the major secretory protein of the luteal phase and first trimester pregnancy endometrium, is not glycosylated prolactin but related to beta-lactoglobulins”. The Journal of Clinical Endocrinology and Metabolism. 65 (5): 1067–71. doi:10.1210/jcem-65-5-1067. PMID 3667877.
- Bell SC, Hales MW, Patel SR, Kirwan PH, Drife JO, Milford-Ward A (Sep 1986). “Amniotic fluid concentrations of secreted pregnancy-associated endometrial alpha 1- and alpha 2-globulins (alpha 1- and alpha 2-PEG)”. British Journal of Obstetrics and Gynaecology. 93 (9): 909–15. doi:10.1111/j.1471-0528.1986.tb08007.x. PMID 3768286. S2CID 70522186.
- Joshi SG, Smith RA, Stokes DK (Nov 1980). “A progestagen-dependent endometrial protein in human amniotic fluid”. Journal of Reproduction and Fertility. 60 (2): 317–21. doi:10.1530/jrf.0.0600317. PMID 6776278.
- Horne CH, Paterson WF, Sutcliffe RG (Jul 1982). “Localization of alpha-uterine protein in human endometrium”. Journal of Reproduction and Fertility. 65 (2): 447–50. doi:10.1530/jrf.0.0650447. PMID 7047733.
Leave a Reply