Albumen prints and egg whites…all the rage back in the day…and a few other things

The albumen print, also called albumen silver print, was published in January 1847 by Louis Désiré Blanquart-Evrard, and was the first commercially exploitable method of producing a photographic print on a paper base from a negative. It used the albumen found in egg whites to bind the photographic chemicals to the paper and became the dominant form of photographic positives from 1855 to the start of the 20th century, with a peak in the 1860–90 period. During the mid-19th century, the carte de visite became one of the more popular uses of the albumen method. In the 19th century, E. & H. T. Anthony & Company were the largest makers and distributors of albumen photographic prints and paper in the United States.
- Blanquart-Evrard, Louis-Désiré (1869). La photographie, ses origines, ses progrès, ses transformations (in French). Lille, France: L. Danel.
- Newhall, Beaumont (April 1955). “60,000 Eggs A Day” (PDF). Image, Journal of Photography of George Eastman House. Rochester, N.Y.: International Museum of Photography at George Eastman House Inc. IV (4): 25–26. Archived from the original (PDF) on 4 March 2016. Retrieved 20 July 2014.
- Welling, William. Photography in America (1978 & 1987)
Creation process

- A piece of paper, usually 100% cotton, is coated with an emulsion of egg white (albumen) and salt (sodium chloride or ammonium chloride), then dried. The albumen seals the paper and creates a slightly glossy surface for the sensitizer to rest on.
- The paper is then dipped in a solution of silver nitrate and water which renders the surface sensitive to UV light.
- The paper is then dried in the absence of UV light.
- The dried, prepared paper is placed in a frame in direct contact under a negative. The negative is traditionally a glass negative with collodion emulsion, but this step can be performed with a modern silver halide negative, too. The paper with negative is then exposed to light until the image achieves the desired level of darkness, which is typically a little lighter than the end product. The progress of the print can be checked during the exposure as it is a printing-out process and the image can be seen taking form as it is being exposed to light. Though direct sunlight was used long ago, a UV exposure unit is often used contemporarily because it is more predictable, as the paper is most sensitive to ultraviolet light.
- A bath of sodium thiosulfate fixes the print’s exposure, preventing further darkening.
- Optional gold or selenium toning improves the photograph’s tone and stabilizes against fading. Depending on the toner, toning may be performed before or after fixing the print.

Because the image emerges as a direct result of exposure to light, without the aid of a developing solution, an albumen print may be said to be a printed rather than a developed photograph.
The table salt (sodium chloride) in the albumen emulsion forms silver chloride when in contact with silver nitrate. Silver chloride is unstable when exposed to light, which makes it decompose into silver and chlorine. The silver ion (Ag+) is reduced to silver (Ag) by addition of an electron during the development/printing process, and the remaining silver chloride is washed out during fixing. The black parts of the image are formed by metallic silver (Ag).
Egg Whites
Local links concerning the egg
Local links concerning egg white
Egg white is the clear liquid (also called the albumen or the glair/glaire) contained within an egg. In chickens it is formed from the layers of secretions of the anterior section of the hen’s oviduct during the passage of the egg. It forms around fertilized or unfertilized egg yolks. The primary natural purpose of egg white is to protect the yolk and provide additional nutrition for the growth of the embryo (when fertilized). Egg white consists primarily of about 90% water into which about 10% proteins (including albumins, mucoproteins, and globulins) are dissolved. Unlike the yolk, which is high in lipids (fats), egg white contains almost no fat, and carbohydrate content is less than 1%. Egg whites contain about 56% of the protein in the egg. Egg white has many uses in food (e.g. meringue, mousse) as well as many other uses (e.g. in the preparation of vaccines such as those for influenza).
- Ornithology, Volume 1994 By Frank B. Gill p. 361
- James, John M.; Zeiger, Robert S.; Lester, Mitchell R.; Fasano, Mary Beth; Gern, James E.; Mansfield, Lyndon E.; Schwartz, Howard J.; Sampson, Hugh A.; Windom, Hugh H.; Machtinger, Steven B.; Lensing, Shelly (1998). “Safe administration of influenza vaccine to patients with egg allergy”. The Journal of Pediatrics. 133 (5): 624–8. doi:10.1016/S0022-3476(98)70101-5. PMID 9821418.
Composition
Egg white makes up around two-thirds of a chicken egg by weight. Water constitutes about 90% of this, with protein, trace minerals, fatty material, vitamins, and glucose contributing the remainder. A raw U.S. large egg contains around 33 grams of egg white with 3.6 grams of protein, 0.24 grams of carbohydrate and 55 milligrams of sodium. It contains no cholesterol and the energy content is about 17 calories. Egg white is an alkaline solution and contains around 149 proteins.[full citation needed] The table below lists the major proteins in egg whites by percentage and their natural functions.[page needed]
- McGee, Harold. On Food and Cooking: The Science and Lore of the Kitchen. New York: Scribner, 2004, edited by Vinay.[page needed]
- Exploratorium
- Takehiko Yamamoto, Mujo Kim (1996-12-13), Hen eggs, ISBN 9780849340055
| Protein | Abundance |
|---|---|
| Ovalbumin | 54% |
| Ovotransferrin | 12% |
| Ovomucoid | 11% |
| Ovoglobulin G2 | 4% |
| Ovoglobulin G3 | 4% |
| Ovomucin | 3.5% |
| Lysozyme | 3.4% |
| Ovoinhibitor | 1.5% |
| Ovoglycoprotein | 1% |
| Flavoprotein | 0.8% |
| Ovomacroglobulin | 0.5% |
| Avidin | 0.05% |
| Cystatin | 0.05% |
Ovalbumin is the most abundant protein in albumen. Classed as phosphoglycoprotein, during storage, it converts into s-ovalbumin (5% at the time of laying) and can reach up to 80% after six months of cold storage. Ovalbumin in solution is heat-resistant. Denaturation temperature is around 84°C, but it can be easily denatured by physical stresses. Conalbumin/ovotransferrin is a glycoprotein which has the capacity to bind the bi- and trivalent metal cations into a complex and is more heat sensitive than ovalbumin. At its isoelectric pH (6.5), it can bind two cations and assume a red or yellow color. These metal complexes are more heat stable than the native state. Ovomucoid is the major allergen from egg white and is a heat-resistant glycoprotein found to be a trypsin inhibitor. Lysozyme is a holoprotein which can lyse the wall of certain Gram-positive bacteria and is found at high levels in the chalaziferous layer and the chalazae which anchor the yolk towards the middle of the egg. Ovomucin is a glycoprotein which may contribute to the gel-like structure of thick albumen. The amount of ovomucin in the thick albumen is four times as great as in the thin albumen.[citation needed]
Foam

The physical stress of beating egg whites can create a foam. Two types of physical stress are caused by beating them with a whisk: denaturation and coagulation.
Denaturation occurs as the whisk drags the liquid through itself, creating a force that unfolds the protein molecules.
Coagulation comes from the mixing of air into the whites, which causes the proteins to come out of their natural state. These denatured proteins gather together where the air and water meet and create multiple bonds with the other unraveled proteins, and thus become a foam, holding the incorporated air in place, because the proteins consist of amino acids; some are hydrophilic (attracted to water) and some are hydrophobic (repelled by water).
- McGee, Harold. On Food and Cooking: The Science and Lore of the Kitchen. New York: Scribner, 2004, edited by Vinay.[page needed]
- “Science of Cooking: Ask the Inquisitive Cooks!”. exploratorium.edu.
When beating egg whites, they are classified in three stages according to the peaks they form when the beater is lifted: soft, firm, and stiff peaks. Overbeaten eggs take on a dry appearance, and eventually collapse. Egg whites do not beat up correctly if they are exposed to any form of fat, such as cooking oils or the fats contained in egg yolk.

Copper bowls have been used in France since the 18th century to stabilize egg foams. The copper in the bowl assists in creating a tighter bond in reactive sulfur items such as egg whites. The bond created is so tight that the sulfurs are prevented from reacting with any other material. A silver–plated bowl has the same result as the copper bowl, as will a pinch of powdered copper supplement from a health store used in a glass bowl. Drawbacks of the copper bowl include the expense of the bowl itself, and that the bowls are difficult to keep clean. Copper contamination from the bowl is minimal, as a cup of foam contains a tenth of a human’s normal daily intake level.
- McGee, Harold. On Food and Cooking: The Science and Lore of the Kitchen. New York: Scribner, 2004, edited by Vinay.[page needed]
- McGee, Harold J.; Long, Sharon R.; Briggs, Winslow R. (1984). “Why whip egg whites in copper bowls?”. Nature. 308 (5960): 667–8. Bibcode:1984Natur.308..667M. doi:10.1038/308667a0. S2CID 4372579.
Health issues
Although egg whites are prized as a source of low-fat, high-protein nutrition, a small number of people cannot eat them. Egg allergy is more common among infants than adults, and most children will outgrow it by the age of five. Allergic reactions against egg white are more common than reactions against egg yolks. In addition to true allergic reactions, some people experience a food intolerance to egg whites.
- “Egg Allergy Facts” Archived 2013-01-12 at the Wayback Machine Asthma and Allergy Foundation of America
- Arnaldo Cantani (2008). Pediatric Allergy, Asthma and Immunology. Berlin: Springer. pp. 710–713. ISBN 978-3-540-20768-9.
Egg allergy is an immune hypersensitivity to proteins found in chicken eggs, and possibly goose, duck, or turkey eggs. Symptoms can be either rapid or gradual in onset. The latter can take hours to days to appear. The former may include anaphylaxis, a potentially life-threatening condition which requires treatment with epinephrine. Other presentations may include atopic dermatitis or inflammation of the esophagus.
- Caubet JC, Wang J (2011). “Current understanding of egg allergy”. Pediatr. Clin. North Am. 58 (2): 427–43. doi:10.1016/j.pcl.2011.02.014. PMC 3069662. PMID 21453811.
- National Report of the Expert Panel on Food Allergy Research, NIH-NIAID 2003 “June 30 2003.pdf” (PDF). Archived from the original (PDF) on 2006-10-04. Retrieved 2006-08-07.
In the United States, 90% of allergic responses to foods are caused by cow’s milk, eggs, wheat, shellfish, peanuts, tree nuts, fish, and soybeans. The declaration of the presence of trace amounts of allergens in foods is not mandatory in any country, with the exception of Brazil.
- “Food Allergy Facts” Archived 2012-10-06 at the Wayback Machine Asthma and Allergy Foundation of America
- Allen KJ, Turner PJ, Pawankar R, Taylor S, Sicherer S, Lack G, Rosario N, Ebisawa M, Wong G, Mills EN, Beyer K, Fiocchi A, Sampson HA (2014). “Precautionary labelling of foods for allergen content: are we ready for a global framework?”. World Allergy Organ J. 7 (1): 1–14. doi:10.1186/1939-4551-7-10. PMC 4005619. PMID 24791183.
- FDA (18 December 2017). “Food Allergies: What You Need to Know”. Food and Drug Administration. Retrieved 12 January 2018.
- “Agência Nacional de Vigilância Sanitária Guia sobre Programa de Controle de Alergênicos” (in Portuguese). Agência Nacional de Vigilância Sanitária (ANVISA). 2016. Archived from the original on 29 April 2018. Retrieved 7 April 2018.
Prevention is by avoiding eating eggs and foods that may contain eggs, such as cake or cookies. It is unclear if the early introduction of the eggs to the diet of babies aged 4–6 months decreases the risk of egg allergies.
- Ierodiakonou D, Garcia-Larsen V, Logan A, Groome A, Cunha S, Chivinge J, Robinson Z, Geoghegan N, Jarrold K, Reeves T, Tagiyeva-Milne N, Nurmatov U, Trivella M, Leonardi-Bee J, Boyle RJ (2016). “Timing of Allergenic Food Introduction to the Infant Diet and Risk of Allergic or Autoimmune Disease: A Systematic Review and Meta-analysis”. JAMA. 316 (11): 1181–1192. doi:10.1001/jama.2016.12623. hdl:10044/1/40479. PMID 27654604.
- Fiocchi A, Dahdah L, Bahna SL, Mazzina O, Assa’ad A (2016). “Doctor, when should I feed solid foods to my infant?”. Curr Opin Allergy Clin Immunol. 16 (4): 404–11. doi:10.1097/aci.0000000000000291. PMID 27327121. S2CID 36508449.
- Anderson J, Malley K, Snell R (2009). “Is 6 months still the best for exclusive breastfeeding and introduction of solids? A literature review with consideration to the risk of the development of allergies”. Breastfeed Rev. 17 (2): 23–31. PMID 19685855.
- Fiocchi A, Assa’ad A, Bahna S (2006). “Food allergy and the introduction of solid foods to infants: a consensus document. Adverse Reactions to Foods Committee, American College of Allergy, Asthma and Immunology”. Ann. Allergy Asthma Immunol. 97 (1): 10–20, quiz 21, 77. doi:10.1016/s1081-1206(10)61364-6. PMID 16892776.
- Caubet JC, Wang J (2011). “Current understanding of egg allergy”. Pediatr. Clin. North Am. 58 (2): 427–43. doi:10.1016/j.pcl.2011.02.014. PMC 3069662. PMID 21453811.
Egg allergy appears mainly in children but can persist into adulthood. In the United States, it is the second most common food allergy in children after cow’s milk. Most children outgrow egg allergy by the age of five, but some people remain allergic for a lifetime. In North America and Western Europe, egg allergy occurs in 0.5% to 2.5% of children under the age of five years. The majority grow out of it by school age, but for roughly one-third, the allergy persists into adulthood. Strong predictors for adult-persistence are anaphylaxis, high egg-specific serum immunoglobulin E (IgE), robust response to the skin prick test and absence of tolerance to egg-containing baked foods.
- Caubet JC, Wang J (2011). “Current understanding of egg allergy”. Pediatr. Clin. North Am. 58 (2): 427–43. doi:10.1016/j.pcl.2011.02.014. PMC 3069662. PMID 21453811.
- Hasan SA, Wells RD, Davis CM (2013). “Egg hypersensitivity in review”. Allergy Asthma Proc. 34 (1): 26–32. doi:10.2500/aap.2013.34.3621. PMID 23406934.
- Urisu A, Kondo Y, Tsuge I (2015). “Hen’s Egg Allergy”. Food Allergy: Molecular Basis and Clinical Practice. Chemical Immunology and Allergy. Vol. 101. pp. 124–30. doi:10.1159/000375416. ISBN 978-3-318-02340-4. PMID 26022872.
- Urisu A, Ebisawa M, Ito K, Aihara Y, Ito S, Mayumi M, Kohno Y, Kondo N (2014). “Japanese Guideline for Food Allergy 2014”. Allergol Int. 63 (3): 399–419. doi:10.2332/allergolint.14-RAI-0770. PMID 25178179.
- “Egg Allergy Facts” Archived 2013-01-12 at the Wayback Machine Asthma and Allergy Foundation of America
Signs and symptoms
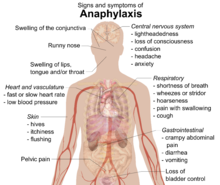
Food allergies usually have an onset from minutes to one to two hours. Symptoms may include: rash, hives, itching of mouth, lips, tongue, throat, eyes, skin, or other areas, swelling of lips, tongue, eyelids, or the whole face, difficulty swallowing, runny or congested nose, hoarse voice, wheezing, shortness of breath, diarrhea, abdominal pain, lightheadedness, fainting, nausea, or vomiting. Symptoms of allergies vary from person to person and may vary from incident to incident. Serious danger regarding allergies can begin when the respiratory tract or blood circulation is affected. The former can be indicated by wheezing, a blocked airway and cyanosis, the latter by weak pulse, pale skin, and fainting. When these symptoms occur the allergic reaction is called anaphylaxis. Anaphylaxis occurs when IgE antibodies are involved, and areas of the body that are not in direct contact with the food become affected and show severe symptoms. Untreated, this can proceed to vasodilation and a low blood pressure situation called anaphylactic shock.
- MedlinePlus Encyclopedia: Food allergy
- Urisu A, Ebisawa M, Ito K, Aihara Y, Ito S, Mayumi M, Kohno Y, Kondo N (2014). “Japanese Guideline for Food Allergy 2014”. Allergol Int. 63 (3): 399–419. doi:10.2332/allergolint.14-RAI-0770. PMID 25178179.
- Sicherer SH, Sampson HA (2014). “Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment”. J Allergy Clin Immunol. 133 (2): 291–307. doi:10.1016/j.jaci.2013.11.020. PMID 24388012.
Young children may exhibit dermatitis/eczema on face, scalp and other parts of the body, in older children knees and elbows are more commonly affected. Children with dermatitis are at greater than expected risk of also exhibiting asthma and allergic rhinitis.
- Pols DH, Wartna JB, van Alphen EI, Moed H, Rasenberg N, Bindels PJ, Bohnen AM (2015). “Interrelationships between Atopic Disorders in Children: A Meta-Analysis Based on ISAAC Questionnaires”. PLOS ONE. 10 (7): e0131869. Bibcode:2015PLoSO..1031869P. doi:10.1371/journal.pone.0131869. PMC 4489894. PMID 26135565.
Causes
Eating egg
The cause is typically the eating of eggs or foods that contain eggs. Briefly, the immune system over-reacts to proteins found in eggs. This allergic reaction may be triggered by small amounts of egg, even egg incorporated into cooked foods, such as cake. People with an allergy to chicken eggs may also be reactive to goose, duck, or turkey eggs.
- Caubet JC, Wang J (2011). “Current understanding of egg allergy”. Pediatr. Clin. North Am. 58 (2): 427–43. doi:10.1016/j.pcl.2011.02.014. PMC 3069662. PMID 21453811.
Vaccines
Influenza vaccines are created by injecting a live virus into fertilized chicken eggs. The viruses are harvested, killed and purified, but a residual amount of egg white protein remains. For adults ages 18 and older there is an option to receive recombinant flu vaccines (RIV3 or RIV4) which are grown on mammalian cell cultures instead of in eggs, and so are no risk for people with severe egg allergy. Recommendations are that for people with a history of mild egg allergy should receive any IIV or RIV vaccine. People with a more severe allergic reaction may also receive any IIV or RIV, but in an inpatient or outpatient medical setting, administered by a healthcare provider. People with a known severe allergic reaction to influenza vaccine (which could be egg protein or the gelatin or the neomycin components of the vaccine) should not receive a flu vaccine.
- “Recommendations for the production and control of influenza vaccine (inactivated)” (PDF). World Health Organization. Archived (PDF) from the original on October 28, 2013. Retrieved May 27, 2013.
- Grohskopf LA, Sokolow LZ, Broder KR; et al. (2017). “Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2017–18 Influenza Season”. MMWR Recomm Rep. 66 (2): 1–20. doi:10.15585/mmwr.rr6602a1. PMC 5837399. PMID 28841201.
Each year the American Academy of Pediatrics (AAP) publishes recommendations for prevention and control of influenza in children. In the 2016-2017 guidelines a change was made, that children with a history of egg allergy may receive the IIV3 or IIV4 vaccine without special precautions. It did, however, state that “Standard vaccination practice should include the ability to respond to acute hypersensitivity reactions.” Prior to this, AAP recommended precautions based on egg allergy history: if no history, immunize; if a history of mild reaction, i.e., hives, immunize in a medical setting with healthcare professionals and resuscitative equipment available; if a history of severe reactions, refer to an allergist.
- Committee on Infectious Diseases, American Academy of Pediatrics (2016). “Recommendations for Prevention and Control of Influenza in Children, 2016-2017”. Pediatrics. 138 (4): e20162527. doi:10.1542/peds.2016-2527. PMID 27600320.
- Committee On Infectious Diseases, American Academy of Pediatrics (2015). “Recommendations for Prevention and Control of Influenza in Children, 2015-2016”. Pediatrics. 136 (4): 792–808. doi:10.1542/peds.2015-2920. PMID 26347430.
- Committee on Infectious Diseases, American Academy of Pediatrics (2011). “Recommendations for prevention and control of influenza in children, 2011-2012”. Pediatrics. 128 (4): 813–25. doi:10.1542/peds.2011-2295. PMID 21890834.
The measles and mumps parts of the “MMR vaccine” (for measles, mumps, and rubella) are cultured on chick embryo cell culture and contain trace amounts of egg protein. The amount of egg protein is lower than in influenza vaccines and the risk of an allergic reaction is much lower. One guideline stated that all infants and children should get the two MMR vaccinations, mentioning that “Studies on large numbers of egg-allergic children show there is no increased risk of severe allergic reactions to the vaccines.” Another guideline recommended that if a child has a known medical history of severe anaphylaxis reaction to eggs, then the vaccination should be done in a hospital center, and the child be kept for observation for 60 minutes before being allowed to leave. The second guideline also stated that if there was a severe reaction to the first vaccination – which could have been to egg protein or the gelatin and neomycin components of the vaccine – the second is contraindicated.
- Piquer-Gibert M, Plaza-Martín A, Martorell-Aragonés A, Ferré-Ybarz L, Echeverría-Zudaire L, Boné-Calvo J, Nevot-Falcó S (2007). “Recommendations for administering the triple viral vaccine and anti-influenza vaccine in patients with egg allergy”. Allergol Immunopathol (Madr). 35 (5): 209–12. doi:10.1157/13110316. PMID 17923075. S2CID 10902757.
- Clark AT, Skypala I, Leech SC, Ewan PW, Dugué P, Brathwaite N, Huber PA, Nasser SM (2010). “British Society for Allergy and Clinical Immunology guidelines for the management of egg allergy”. Clin. Exp. Allergy. 40 (8): 1116–29. doi:10.1111/j.1365-2222.2010.03557.x. PMID 20649608. S2CID 29950268.
Exercise as a contributing factor
There is a condition called food-dependent, exercise-induced anaphylaxis (FDEIAn). Exercise can trigger hives and more severe symptoms of an allergic reaction. For some people with this condition, exercise alone is not sufficient, nor consumption of a food to which they are mildly allergic sufficient, but when the food in question is consumed within a few hours before high intensity exercise, the result can be anaphylaxis. Egg are specifically mentioned as a causative food. One theory is that exercise is stimulating the release of mediators such as histamine from IgE-activated mast cells. Two of the reviews postulate that exercise is not essential for the development of symptoms, but rather that it is one of several augmentation factors, citing evidence that the culprit food in combination with alcohol or aspirin will result in a respiratory anaphylactic reaction.
- Feldweg AM (2017). “Food-Dependent, Exercise-Induced Anaphylaxis: Diagnosis and Management in the Outpatient Setting”. J Allergy Clin Immunol Pract. 5 (2): 283–288. doi:10.1016/j.jaip.2016.11.022. PMID 28283153.
- Pravettoni V, Incorvaia C (2016). “Diagnosis of exercise-induced anaphylaxis: current insights”. J Asthma Allergy. 9: 191–198. doi:10.2147/JAA.S109105. PMC 5089823. PMID 27822074.
- Kim CW, Figueroa A, Park CH, Kwak YS, Kim KB, Seo DY, Lee HR (2013). “Combined effects of food and exercise on anaphylaxis”. Nutr Res Pract. 7 (5): 347–51
Mechanisms
Conditions caused by food allergies are classified into three groups according to the mechanism of the allergic response:
- IgE-mediated (classic) – the most common type, manifesting acute changes that occur shortly after eating, and may progress to anaphylaxis
- Non-IgE mediated – characterized by an immune response not involving immunoglobulin E; may occur hours to days after eating, complicating diagnosis
- IgE and non-IgE-mediated – a hybrid of the above two types
- “Food allergy”. NHS Choices. 16 May 2016. Retrieved 31 January 2017.
A food allergy is when the body’s immune system reacts unusually to specific foods
Allergic reactions are hyperactive responses of the immune system to generally innocuous substances, such as proteins in the foods we eat. Why some proteins trigger allergic reactions while others do not is not entirely clear, although in part thought to be due to resistance to digestion. Because of this, intact or largely intact proteins reach the small intestine, which has a large presence of white blood cells involved in immune reactions. The heat of cooking structurally degrades protein molecules, potentially making them less allergenic.
- Food Reactions. Allergies Archived 2010-04-16 at the Wayback Machine. Foodreactions.org. Kent, England. 2005. Accessed 27 Apr 2010.
- Davis PJ, Williams SC (1998). “Protein modification by thermal processing”. Allergy. 53 (46 Suppl): 102–5. doi:10.1111/j.1398-9995.1998.tb04975.x. PMID 9826012. S2CID 10621652.
- Verhoeckx KC, Vissers YM, Baumert JL, Faludi R, Feys M, Flanagan S, Herouet-Guicheney C, Holzhauser T, Shimojo R, van der Bolt N, Wichers H, Kimber I (June 2015). “Food processing and allergenicity”. Food Chem Toxicol. 80: 223–240. doi:10.1016/j.fct.2015.03.005. PMID 25778347.
The pathophysiology of allergic responses can be divided into two phases. The first is an acute response that occurs within minutes to an hour or two exposure to an allergen. This phase can either subside or progress into a “late-phase reaction” which can substantially prolong the symptoms of a response, and result in more tissue damage. In the early stages of acute allergic reaction, lymphocytes previously sensitized to a specific protein or protein fraction react by quickly producing a particular type of antibody known as secreted IgE (sIgE), which circulates in the blood and binds to IgE-specific receptors on the surface of other kinds of immune cells called mast cells and basophils. Both of these are involved in the acute inflammatory response. Activated mast cells and basophils undergo a process called degranulation, during which they release histamine and other inflammatory chemical mediators called (cytokines, interleukins, leukotrienes, and prostaglandins) into the surrounding tissue causing several systemic effects, such as vasodilation, mucous secretion, nerve stimulation, and smooth-muscle contraction. This results in runny nose, itchiness, shortness of breath, and potentially anaphylaxis. Depending on the individual, the allergen, and the mode of introduction, the symptoms can be system-wide (classical anaphylaxis), or localized to particular body systems; asthma is localized to the respiratory system while eczema is localized to the skin.
- Janeway, Charles; Paul Travers; Mark Walport; Mark Shlomchik (2001). Immunobiology; Fifth Edition. New York and London: Garland Science. pp. e–book. ISBN 978-0-8153-4101-7. Archived from the original on 2009-06-28.
After the chemical mediators of the acute response subside, late-phase responses can often occur due to the migration of other white blood cells such as neutrophils, lymphocytes, eosinophils, and macrophages to the initial reaction sites. This is usually seen 2–24 hours after the original reaction. Cytokines from mast cells may also play a role in the persistence of long-term effects. Late-phase responses seen in asthma are slightly different from those seen in other allergic responses, although they are still caused by release of mediators from eosinophils.
- Grimbaldeston MA, Metz M, Yu M, Tsai M, Galli SJ (2006). “Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune responses”. Curr. Opin. Immunol. 18 (6): 751–60. doi:10.1016/j.coi.2006.09.011. PMID 17011762.
- Holt PG, Sly PD (2007). “Th2 cytokines in the asthma late-phase response”. Lancet. 370 (9596): 1396–8. doi:10.1016/S0140-6736(07)61587-6. PMID 17950849. S2CID 40819814.
Five major allergenic proteins from the egg of the domestic chicken (Gallus domesticus) have been identified; these are designated Gal d 1–5. Four of these are in egg white: ovomucoid (Gal d 1), ovalbumin (Gal d 2), ovotransferrin (Gal d 3) and lysozyme (Gal d 4). Of these, ovomucoid is the dominant allergen, and one that is less likely to be outgrown as children get older. Ingestion of under-cooked egg may trigger more severe clinical reactions than well-cooked egg. In egg yolk, alpha-livetin (Gal d 5) is the major allergen, but various vitellins may also trigger a reaction. People allergic to alpha-livetin may experience respiratory symptoms such as rhinitis and/or asthma when exposed to chickens, because the yolk protein is also found in live birds. In addition to IgE-mediated responses, egg allergy can manifest as atopic dermatitis, especially in infants and young children. Some will display both, so that a child could react to an oral food challenge with allergic symptoms, followed a day or two later with a flare up of atopic dermatitis and/or gastrointestinal symptoms, including allergic eosinophilic esophagitis.
- Caubet JC, Wang J (2011). “Current understanding of egg allergy”. Pediatr. Clin. North Am. 58 (2): 427–43. doi:10.1016/j.pcl.2011.02.014. PMC 3069662. PMID 21453811.
- Urisu A, Kondo Y, Tsuge I (2015). “Hen’s Egg Allergy”. Food Allergy: Molecular Basis and Clinical Practice. Chemical Immunology and Allergy. Vol. 101. pp. 124–30. doi:10.1159/000375416. ISBN 978-3-318-02340-4. PMID 26022872.
Non-allergic intolerance
Egg whites, which are potentially histamine liberators, also provoke a nonallergic response in some people. In this situation, proteins in egg white directly trigger the release of histamine from mast cells. Because this mechanism is classified as a pharmacological reaction, or “pseudoallergy“, the condition is considered a food intolerance instead of a true immunoglobulin E (IgE) based allergic reaction.
- Arnaldo Cantani (2008). Pediatric Allergy, Asthma and Immunology. Berlin: Springer. pp. 710–713. ISBN 978-3-540-20768-9.
- Joris, Isabelle; Majno, Guido (2004). Cells, tissues, and disease: principles of general pathology. Oxford [Oxfordshire]: Oxford University Press. p. 538. ISBN 978-0-19-514090-3.
The response is usually localized, typically in the gastrointestinal tract. Symptoms may include abdominal pain, diarrhea, or any other symptoms typical to histamine release. If sufficiently strong, it can result in an anaphylactoid reaction, which is clinically indistinguishable from true anaphylaxis. Some people with this condition tolerate small quantities of egg whites. They are more often able to tolerate well-cooked eggs, such as found in cake or dried egg-based pasta, than incompletely cooked eggs, such as fried eggs or meringues, or uncooked eggs.
- Arnaldo Cantani (2008). Pediatric Allergy, Asthma and Immunology. Berlin: Springer. pp. 710–713. ISBN 978-3-540-20768-9.
- Joris, Isabelle; Majno, Guido (2004). Cells, tissues, and disease: principles of general pathology. Oxford [Oxfordshire]: Oxford University Press. p. 538. ISBN 978-0-19-514090-3.
- Carina Venter; Isabel Skypala (2009). Food Hypersensitivity: Diagnosing and Managing Food Allergies and Intolerance. Wiley-Blackwell. pp. 129–131. ISBN 978-1-4051-7036-9.
Diagnosis
Diagnosis of egg allergy is based on the person’s history of allergic reactions, skin prick test (SPT), patch test and measurement of egg-specific serum immunoglobulin E (IgE or sIgE). Confirmation is by double-blind, placebo-controlled food challenges. SPT and sIgE have sensitivity greater than 90% but specificity in the 50-60% range, meaning these tests will detect an egg sensitivity, but will also be positive for other allergens. For young children, attempts have been made to identify SPT and sIgE responses strong enough to avoid the need for a confirming oral food challenge.
- Soares-Weiser K, Takwoingi Y, Panesar SS, Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Halken S, Poulsen L, van Ree R, Vlieg-Boerstra BJ, Sheikh A (2014). “The diagnosis of food allergy: a systematic review and meta-analysis”. Allergy. 69 (1): 76–86. doi:10.1111/all.12333. PMID 24329961.
- Calvani M, Arasi S, Bianchi A, Caimmi D, Cuomo B, Dondi A, Indirli GC, La Grutta S, Panetta V, Verga MC (2015). “Is it possible to make a diagnosis of raw, heated, and baked egg allergy in children using cutoffs? A systematic review”. Pediatr Allergy Immunol. 26 (6): 509–21. doi:10.1111/pai.12432. hdl:10447/143352. PMID 26102461. S2CID 206241392.
- Hasan SA, Wells RD, Davis CM (2013). “Egg hypersensitivity in review”. Allergy Asthma Proc. 34 (1): 26–32. doi:10.2500/aap.2013.34.3621. PMID 23406934.
- Urisu A, Kondo Y, Tsuge I (2015). “Hen’s Egg Allergy”. Food Allergy: Molecular Basis and Clinical Practice. Chemical Immunology and Allergy. Vol. 101. pp. 124–30. doi:10.1159/000375416. ISBN 978-3-318-02340-4. PMID 26022872
Prevention
When eggs are introduced to a baby’s diet is thought to affect risk of developing allergy, but there are contradictory recommendations. A 2016 review acknowledged that introducing peanuts early appears to have a benefit, but stated “The effect of early introduction of egg on egg allergy are controversial.” A meta-analysis published the same year supported the theory that early introduction of eggs into an infant’s diet lowers risk, and a review of allergens in general stated that introducing solid foods at 4–6 months may result in the lowest subsequent allergy risk. However, an older consensus document from the American College of Allergy, Asthma and Immunology recommended that introduction of chicken eggs be delayed to 24 months of age.
- Ierodiakonou D, Garcia-Larsen V, Logan A, Groome A, Cunha S, Chivinge J, Robinson Z, Geoghegan N, Jarrold K, Reeves T, Tagiyeva-Milne N, Nurmatov U, Trivella M, Leonardi-Bee J, Boyle RJ (2016). “Timing of Allergenic Food Introduction to the Infant Diet and Risk of Allergic or Autoimmune Disease: A Systematic Review and Meta-analysis”. JAMA. 316 (11): 1181–1192. doi:10.1001/jama.2016.12623. hdl:10044/1/40479. PMID 27654604.
- Fiocchi A, Dahdah L, Bahna SL, Mazzina O, Assa’ad A (2016). “Doctor, when should I feed solid foods to my infant?”. Curr Opin Allergy Clin Immunol. 16 (4): 404–11. doi:10.1097/aci.0000000000000291. PMID 27327121. S2CID 36508449.
- Anderson J, Malley K, Snell R (2009). “Is 6 months still the best for exclusive breastfeeding and introduction of solids? A literature review with consideration to the risk of the development of allergies”. Breastfeed Rev. 17 (2): 23–31. PMID 19685855.
- Fiocchi A, Assa’ad A, Bahna S (2006). “Food allergy and the introduction of solid foods to infants: a consensus document. Adverse Reactions to Foods Committee, American College of Allergy, Asthma and Immunology”. Ann. Allergy Asthma Immunol. 97 (1): 10–20, quiz 21, 77. doi:10.1016/s1081-1206(10)61364-6. PMID 16892776.
Treatment

The mainstay of treatment is total avoidance of egg protein intake. This is complicated because the declaration of the presence of trace amounts of allergens in foods is not mandatory (see regulation of labelling).
- Martorell A, Alonso E, Boné J, Echeverría L, López MC, Martín F, et al. (2013). “Position document: IgE-mediated allergy to egg protein”. Allergol Immunopathol (Madr) (Review). 41 (5): 320–36. doi:10.1016/j.aller.2013.03.005. PMID 23830306.
Treatment for accidental ingestion of egg products by allergic individuals varies depending on the sensitivity of the person. An antihistamine such as diphenhydramine (Benadryl) may be prescribed. Sometimes prednisone will be prescribed to prevent a possible late phase Type I hypersensitivity reaction. Severe allergic reactions (anaphalaxis) may require treatment with an epinephrine pen, an injection device designed to be used by a non-healthcare professional when emergency treatment is warranted.
- Tang AW (2003). “A practical guide to anaphylaxis”. Am Fam Physician. 68 (7): 1325–1332. PMID 14567487.
- The EAACI Food Allergy and Anaphylaxis Guidelines Group (August 2014). “Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology”. Allergy. 69 (8): 1026–45. doi:10.1111/all.12437. PMID 24909803. S2CID 11054771.
Immunotherapy
There is active research on trying oral immunotherapy (OIT) to desensitize people to egg allergens. A Cochrane Review concluded that OIT can desensitize people, but it remains unclear whether long-term tolerance develops after treatment ceases, and 69% of the people enrolled in the trials had adverse effects. They concluded there was a need for standardized protocols and guidelines prior to incorporating OIT into clinical practice. A second review noted that allergic reactions, up to anaphylaxis, can occur during OIT, and recommends this treatment not be routine medical practice. A third review limited its scope to trials of baked egg-containing goods such as bread or cake as a means of resolving egg allergy. Again, there were some successes, but also some severe allergic reactions, and the authors came down on the side of not recommending this as treatment.
- Romantsik, O; Tosca, MA; Zappettini, S; Calevo, MG (20 April 2018). “Oral and sublingual immunotherapy for egg allergy”. The Cochrane Database of Systematic Reviews. 2018 (4): CD010638. doi:10.1002/14651858.CD010638.pub3. PMC 6494514. PMID 29676439.
- Ibáñez MD, Escudero C, Sánchez-García S, Rodríguez del Río P (2015). “Comprehensive Review of Current Knowledge on Egg Oral Immunotherapy”. J Investig Allergol Clin Immunol. 25 (5): 316–28, quiz 2 p following 328. PMID 26727760.
- Lambert R, Grimshaw KE, Ellis B, Jaitly J, Roberts G (2017). “Evidence that eating baked egg or milk influences egg or milk allergy resolution: a systematic review”. Clin. Exp. Allergy. 47 (6): 829–837. doi:10.1111/cea.12940. PMID 28516451. S2CID 5207549.
Avoiding eggs
Prevention of egg-allergic reactions means avoiding eggs and egg-containing foods. People with an allergy to chicken eggs may also be allergic to other types of eggs, such as goose, duck, or turkey eggs. In cooking, eggs are multifunctional: they may act as an emulsifier to reduce oil/water separation (mayonnaise), a binder (water binding and particle adhesion, as in meatloaf), or an aerator (cakes, especially angel food). Some commercial egg substitutes can substitute for particular functions (potato starch and tapioca for water binding, whey protein or bean water for aeration or particle binding, or soy lecithin or avocado for emulsification). Food companies produce egg-free mayonnaise and other replacement foods. Alfred Bird invented egg-free Bird’s Custard, the original version of what is known generically as custard powder today.
- Carey, John (1997). Eyewitness to Science. Harvard University Press. p. 173. ISBN 9780674287556.
- Caubet JC, Wang J (2011). “Current understanding of egg allergy”. Pediatr. Clin. North Am. 58 (2): 427–43. doi:10.1016/j.pcl.2011.02.014. PMC 3069662. PMID 21453811.
Most people find it necessary to strictly avoid any item containing eggs, including:
- Albumin (egg white protein)
- Apovitellin (egg yolk protein)
- Egg Beaters (cholesterol-free, uses egg whites)
- Dried egg solids, powdered egg
- Egg, egg white, egg yolk
- Egg wash
- Eggnog
- Fat substitutes (some)
- Livetin (egg yolk protein)
- Lysozyme (egg white protein)
- Mayonnaise
- Meringue or meringue powder
- Ovalbumin (egg white protein)
- Ovoglobulin (egg white protein)
- Ovomucin (egg white protein)
- Ovomucoid (egg white protein)
- Ovotransferrin (egg white protein)
- Ovovitelia (egg yolk protein)
- Ovovitellin (egg yolk protein)
- Silici albuminate
- Simplesse
- Vitellin (egg yolk protein)
- “Egg Allergy Facts” Archived 2013-01-12 at the Wayback Machine Asthma and Allergy Foundation of America
Ingredients that sometimes include egg protein include: artificial flavoring, natural flavoring, lecithin and nougat candy.
Probiotic products have been tested, and some found to contain milk and egg proteins which were not always indicated on the labels.
- Nanagas, VC; Baldwin, JL; Karamched, KR (July 2017). “Hidden Causes of Anaphylaxis”. Current Allergy and Asthma Reports. 17 (7): 44. doi:10.1007/s11882-017-0713-2. PMID 28577270. S2CID 33691910.
Prognosis
The majority of children outgrow egg allergy. One review reported that 70% of children will outgrow this allergy by 16 years. In subsequently published longitudinal studies, one reported that for 140 infants who had challenge-confirmed egg allergy, 44% had resolved by two years. A second reported that for 203 infants with confirmed IgE-mediated egg allergy, 45% resolved by two years of age, 66% by four years, and 71% by six years. Children will be able to tolerate eggs as an ingredient in baked goods and well-cooked eggs sooner than under-cooked eggs. Resolution was more likely if baseline serum IgE was lower, and if the baseline symptoms did not include anaphylaxis.
- Hasan SA, Wells RD, Davis CM (2013). “Egg hypersensitivity in review”. Allergy Asthma Proc. 34 (1): 26–32. doi:10.2500/aap.2013.34.3621. PMID 23406934.
- Peters RL, Dharmage SC, Gurrin LC, Koplin JJ, Ponsonby AL, Lowe AJ, Tang ML, Tey D, Robinson M, Hill D, Czech H, Thiele L, Osborne NJ, Allen KJ (2014). “The natural history and clinical predictors of egg allergy in the first 2 years of life: a prospective, population-based cohort study”. J. Allergy Clin. Immunol. 133 (2): 485–91. doi:10.1016/j.jaci.2013.11.032. PMID 24373356. S2CID 24028314.
- Arik Yilmaz E, Cavkaytar O, Buyuktiryaki B, Sekerel BE, Soyer O, Sackesen C (2015). “Factors associated with the course of egg allergy in children”. Ann. Allergy Asthma Immunol. 115 (5): 434–438.e1. doi:10.1016/j.anai.2015.08.012. PMID 26505933.
Epidemiology
In countries in North America and western Europe, where use of cow’s milk based infant formula is common, chicken egg allergy is the second most common food allergy in infants and young children after cow’s milk. However, in Japan, egg allergy is first and cow’s milk second, followed by wheat and then the other common allergenic foods. A review from South Africa reported egg and peanut as the two most common allergenic foods.
- Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A (2014). “Prevalence of common food allergies in Europe: a systematic review and meta-analysis”. Allergy. 69 (8): 992–1007. doi:10.1111/all.12423. PMID 24816523. S2CID 28692645.
- Gray CL (2017). “Food Allergy in South Africa”. Curr Allergy Asthma Rep. 17 (6): 35. doi:10.1007/s11882-017-0703-4. PMID 28470372. S2CID 44840606.
- Hasan SA, Wells RD, Davis CM (2013). “Egg hypersensitivity in review”. Allergy Asthma Proc. 34 (1): 26–32. doi:10.2500/aap.2013.34.3621. PMID 23406934.
- Urisu A, Kondo Y, Tsuge I (2015). “Hen’s Egg Allergy”. Food Allergy: Molecular Basis and Clinical Practice. Chemical Immunology and Allergy. Vol. 101. pp. 124–30. doi:10.1159/000375416. ISBN 978-3-318-02340-4. PMID 26022872
- Urisu A, Ebisawa M, Ito K, Aihara Y, Ito S, Mayumi M, Kohno Y, Kondo N (2014). “Japanese Guideline for Food Allergy 2014”. Allergol Int. 63 (3): 399–419. doi:10.2332/allergolint.14-RAI-0770. PMID 25178179.
Incidence and prevalence are terms commonly used in describing disease epidemiology. Incidence is newly diagnosed cases, which can be expressed as new cases per year per million people. Prevalence is the number of cases alive, expressible as existing cases per million people during a period of time. Egg allergies are usually observed in infants and young children, and often disappear with age (see Prognosis), so prevalence of egg allergy may be expressed as a percentage of children under a set age. One review estimates that in North American and western European populations the prevalence of egg allergy in children under the age of five years is 1.8-2.0%. A second described the range in young children as 0.5-2.5%. Although the majority of children develop tolerance as they age into school age years, for roughly one-third the allergy persists into adulthood. Strong predictors for adult-persistent allergy are anaphylactic symptoms as a child, high egg-specific serum IgE, robust response to the skin prick test and absence of tolerance to egg-containing baked foods. Self-reported allergy prevalence is always higher than food-challenge confirmed allergy.
- Hasan SA, Wells RD, Davis CM (2013). “Egg hypersensitivity in review”. Allergy Asthma Proc. 34 (1): 26–32. doi:10.2500/aap.2013.34.3621. PMID 23406934.
- Urisu A, Kondo Y, Tsuge I (2015). “Hen’s Egg Allergy”. Food Allergy: Molecular Basis and Clinical Practice. Chemical Immunology and Allergy. Vol. 101. pp. 124–30. doi:10.1159/000375416. ISBN 978-3-318-02340-4. PMID 26022872
- “What is Prevalence?” National Institute of Mental Health (Accessed 25 December 2020).
- Caubet JC, Wang J (2011). “Current understanding of egg allergy”. Pediatr. Clin. North Am. 58 (2): 427–43. doi:10.1016/j.pcl.2011.02.014. PMC 3069662. PMID 21453811.
For all age groups, a review of fifty studies conducted in Europe estimated 2.5% for self-reported egg allergy and 0.2% for confirmed. National survey data in the United States collected in 2005 and 2006 showed that from age six and older, the prevalence of serum IgE confirmed egg allergy was under 0.2%.
- Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A (2014). “Prevalence of common food allergies in Europe: a systematic review and meta-analysis”. Allergy. 69 (8): 992–1007.
- Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, Massing M, Cohn RD, Zeldin DC (2010). “National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006”. J. Allergy Clin. Immunol. 126 (4): 798–806.e13. doi:10.1016/j.jaci.2010.07.026. PMC 2990684. PMID 20920770.
Adult-onset of egg allergy is rare, but there is confirmation of cases. Some were described as having started in late teenage years; another group were workers in the baking industry who were exposed to powdered egg dust.
- Unsel M, Sin AZ, Ardeniz O, Erdem N, Ersoy R, Gulbahar O, Mete N, Kokuludağ A (2007). “New onset egg allergy in an adult”. J Investig Allergol Clin Immunol. 17 (1): 55–8. PMID 17323866.
Regulation
Whether food allergy prevalence is increasing or not, food allergy awareness has definitely increased, with impacts on the quality of life for children, their parents and their immediate caregivers. In the United States, the Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA) causes people to be reminded of allergy problems every time they handle a food package, and restaurants have added allergen warnings to menus. The Culinary Institute of America, a premier school for chef training, has courses in allergen-free cooking and a separate teaching kitchen. School systems have protocols about what foods can be brought into the school. Despite all these precautions, people with serious allergies are aware that accidental exposure can easily occur at other peoples’ houses, at school or in restaurants.
- Ravid NL, Annunziato RA, Ambrose MA, Chuang K, Mullarkey C, Sicherer SH, Shemesh E, Cox AL (2015). “Mental health and quality-of-life concerns related to the burden of food allergy”. Psychiatr. Clin. North Am. 38 (1): 77–89. doi:10.1016/j.psc.2014.11.004. PMID 25725570.
- Morou Z, Tatsioni A, Dimoliatis ID, Papadopoulos NG (2014). “Health-related quality of life in children with food allergy and their parents: a systematic review of the literature”. J Investig Allergol Clin Immunol. 24 (6): 382–95. PMID 25668890.
- Lange L (2014). “Quality of life in the setting of anaphylaxis and food allergy”. Allergo J Int. 23 (7): 252–260. doi:10.1007/s40629-014-0029-x. PMC 4479473. PMID 26120535.
- van der Velde JL, Dubois AE, Flokstra-de Blok BM (2013). “Food allergy and quality of life: what have we learned?”. Curr Allergy Asthma Rep. 13 (6): 651–61. doi:10.1007/s11882-013-0391-7. PMID 24122150. S2CID 326837.
- Culinary Institute of America Allergen-free oasis comes to the CIA (2017)
- Shah E, Pongracic J (2008). “Food-induced anaphylaxis: who, what, why, and where?”. Pediatr Ann. 37 (8): 536–41. doi:10.3928/00904481-20080801-06. PMID 18751571.
Regulation of labelling

In response to the risk that certain foods pose to those with food allergies, some countries have responded by instituting labeling laws that require food products to clearly inform consumers if their products contain major allergens or byproducts of major allergens among the ingredients intentionally added to foods. Nevertheless, there are no labeling laws to mandatory declare the presence of trace amounts in the final product as a consequence of cross-contamination, except in Brazil.
- Allen KJ, Turner PJ, Pawankar R, Taylor S, Sicherer S, Lack G, Rosario N, Ebisawa M, Wong G, Mills EN, Beyer K, Fiocchi A, Sampson HA (2014). “Precautionary labelling of foods for allergen content: are we ready for a global framework?”. World Allergy Organ J. 7 (1): 1–14. doi:10.1186/1939-4551-7-10. PMC 4005619. PMID 24791183.
- FDA (18 December 2017). “Food Allergies: What You Need to Know”. Food and Drug Administration. Retrieved 12 January 2018.
- “Agência Nacional de Vigilância Sanitária Guia sobre Programa de Controle de Alergênicos” (in Portuguese). Agência Nacional de Vigilância Sanitária (ANVISA). 2016. Archived from the original on 29 April 2018. Retrieved 7 April 2018.
- “Food Allergen Labeling and Consumer Protection Act of 2004”. FDA. 2 August 2004. Retrieved 7 March 2022.
- “Food allergen labelling and information requirements under the EU Food Information for Consumers Regulation No. 1169/2011: Technical Guidance” (April 2015).
- FDA (14 December 2017). “Have Food Allergies? Read the Label”. Food and Drug Administration. Retrieved 14 January 2018.
- “Food Ingredients of Public Health Concern” (PDF). United States Department of Agriculture. Food Safety and Inspection Service. 7 March 2017. Retrieved 16 February 2018.
- “Allergies and Food Safety”. United States Department of Agriculture. Food Safety and Inspection Service. 1 December 2016. Retrieved 16 February 2018.
Ingredients intentionally added
FALCPA became effective 1 January 2006, requiring companies selling foods in the United States to disclose on labels whether a packaged food product contains any of these eight major food allergens, added intentionally: cow’s milk, peanuts, eggs, shellfish, fish, tree nuts, soy and wheat. This list originated in 1999 from the World Health Organisation Codex Alimentarius Commission. To meet FALCPA labeling requirements, if an ingredient is derived from one of the required-label allergens, then it must either have its “food sourced name” in parentheses, for example “Casein (milk),” or as an alternative, there must be a statement separate but adjacent to the ingredients list: “Contains milk” (and any other of the allergens with mandatory labeling).
- “Food Allergen Labeling and Consumer Protection Act of 2004”. FDA. 2 August 2004. Retrieved 7 March 2022.
- FDA (14 December 2017). “Have Food Allergies? Read the Label”. Food and Drug Administration. Retrieved 14 January 2018.
- Allen KJ, Turner PJ, Pawankar R, Taylor S, Sicherer S, Lack G, Rosario N, Ebisawa M, Wong G, Mills EN, Beyer K, Fiocchi A, Sampson HA (2014). “Precautionary labelling of foods for allergen content: are we ready for a global framework?”. World Allergy Organ J. 7 (1): 1–14.
FALCPA applies to packaged foods regulated by the FDA, which does not include poultry, most meats, certain egg products, and most alcoholic beverages. However, some meat, poultry, and egg processed products may contain allergenic ingredients. These products are regulated by the Food Safety and Inspection Service (FSIS), which requires that any ingredient be declared in the labeling only by its common or usual name. Neither the identification of the source of a specific ingredient in a parenthetical statement nor the use of statements to alert for the presence of specific ingredients, like “Contains: milk”, are mandatory according to FSIS. FALCPA also does not apply to food prepared in restaurants. The EU Food Information for Consumers Regulation 1169/2011 – requires food businesses to provide allergy information on food sold unpackaged, for example, in catering outlets, deli counters, bakeries and sandwich bars.
- “Food Ingredients of Public Health Concern” (PDF). United States Department of Agriculture. Food Safety and Inspection Service. 7 March 2017. Retrieved 16 February 2018.
- “Allergies and Food Safety”. United States Department of Agriculture. Food Safety and Inspection Service. 1 December 2016. Retrieved 16 February 2018.
- Roses JB (2011). “Food allergen law and the Food Allergen Labeling and Consumer Protection Act of 2004: falling short of true protection for food allergy sufferers”. Food Drug Law J. 66 (2): 225–42, ii. PMID 24505841.
- FDA (18 July 2006). “Food Allergen Labeling And Consumer Protection Act of 2004 Questions and Answers”. Food and Drug Administration. Retrieved 12 March 2018.
- “Allergy and intolerance: guidance for businesses”. Archived from the original on 2014-12-08. Retrieved 2014-12-12.
- FDA (18 December 2017). “Food Allergies: What You Need to Know”. Food and Drug Administration. Retrieved 12 January 2018.
Trace amounts as a result of cross-contamination
Labeling regulations have been modified to provide for mandatory labeling of ingredients plus voluntary labeling, termed precautionary allergen labeling (PAL), also known as “may contain” statements, for possible, inadvertent, trace amount, cross-contamination during production. PAL labeling can be confusing to consumers, especially as there can be many variations on the wording of the warning. As of 2014 PAL is regulated only in Switzerland, Japan, Argentina, and South Africa. Argentina decided to prohibit precautionary allergen labeling since 2010, and instead puts the onus on the manufacturer to control the manufacturing process and label only those allergenic ingredients known to be in the products. South Africa does not permit the use of PAL, except when manufacturers demonstrate the potential presence of allergen due to cross-contamination through a documented risk assessment and despite adherence to Good Manufacturing Practice. In Australia and New Zealand there is a recommendation that PAL be replaced by guidance from VITAL 2.0 (Vital Incidental Trace Allergen Labeling). A review identified “the eliciting dose for an allergic reaction in 1% of the population” as ED01. This threshold reference dose for foods such as cow’s milk, egg, peanut and other proteins) will provide food manufacturers with guidance for developing precautionary labeling and give consumers a better idea of might be accidentally in a food product beyond “may contain.” VITAL 2.0 was developed by the Allergen Bureau, a food industry sponsored, non-government organization. The European Union has initiated a process to create labeling regulations for unintentional contamination but is not expected to publish such before 2024.
- DunnGalvin A, Chan CH, et al. (2015). “Precautionary allergen labelling: perspectives from key stakeholder groups”. Allergy. 70 (9): 1039–1051. doi:10.1111/all.12614. PMID 25808296. S2CID 18362869.
- Zurzolo GA, de Courten M, Koplin J, Mathai ML, Allen KJ (2016). “Is advising food allergic patients to avoid food with precautionary allergen labelling out of date?”. Curr Opin Allergy Clin Immunol. 16 (3): 272–277. doi:10.1097/ACI.0000000000000262. PMID 26981748. S2CID 21326926.
- Allen KJ, Remington BC, Baumert JL, Crevel RW, Houben GF, Brooke-Taylor S, Kruizinga AG, Taylor SL (2014). “Allergen reference doses for precautionary labeling (VITAL 2.0): clinical implications”. J. Allergy Clin. Immunol. 133 (1): 156–164. doi:10.1016/j.jaci.2013.06.042. PMID 23987796.
- Taylor SL, Baumert JL, Kruizinga AG, Remington BC, Crevel RW, Brooke-Taylor S, Allen KJ, Houben G (2014). “Establishment of Reference Doses for residues of allergenic foods: report of the VITAL Expert Panel”. Food Chem. Toxicol. 63: 9–17. doi:10.1016/j.fct.2013.10.032. PMID 24184597.
- The VITAL Program Allergen Bureau, Australia and New Zealand.
- Popping B, Diaz-Amigo C (2018). “European Regulations for Labeling Requirements for Food Allergens and Substances Causing Intolerances: History and Future”. J AOAC Int. 101 (1): 2–7. doi:10.5740/jaoacint.17-0381. PMID 29202901.
- Allen KJ, Turner PJ, Pawankar R, Taylor S, Sicherer S, Lack G, Rosario N, Ebisawa M, Wong G, Mills EN, Beyer K, Fiocchi A, Sampson HA (2014). “Precautionary labelling of foods for allergen content: are we ready for a global framework?”. World Allergy Organ J. 7 (1): 1–14. doi:10.1186/1939-4551-7-10. PMC 4005619. PMID 24791183.
In Brazil since April 2016, the declaration of the possibility of cross-contamination is mandatory when the product does not intentionally add any allergenic food or its derivatives but the Good Manufacturing Practices and allergen control measures adopted are not sufficient to prevent the presence of accidental trace amounts. These allergens include wheat, rye, barley, oats and their hybrids, crustaceans, eggs, fish, peanuts, soybean, milk of all species of mammalians, almonds, hazelnuts, cashew nuts, Brazil nuts, macadamia nuts, walnuts, pecan nuts, pistaches, pine nuts, and chestnuts.
- “Agência Nacional de Vigilância Sanitária Guia sobre Programa de Controle de Alergênicos” (in Portuguese). Agência Nacional de Vigilância Sanitária (ANVISA). 2016. Archived from the original on 29 April 2018. Retrieved 7 April 2018.
See also
- List of allergens (food and non-food)
Eggs are susceptible to Salmonella contamination. Thorough cooking eliminates the direct threat (i.e. cooked egg whites that are solid and not runny), but the threat of cross-contamination remains if people handle contaminated eggs and then touch other foods or items in the kitchen, thus spreading the bacteria. In August 2010, the FDA ordered the recall of 380 million eggs because of possible Salmonella contamination.
- Roan, Shari (August 20, 2010). “Eggs and salmonella: What you need to know”. Los Angeles Times. Retrieved September 14, 2011.
Cooked eggs are a good source of biotin. However, daily consumption of raw egg whites for several months may result in biotin deficiency, due to their avidin content, as the avidin tightly binds biotin and prevents its absorption.
- Dasgupta, Amitava (2019-01-01), Dasgupta, Amitava (ed.), “Chapter 2 – Biotin: Pharmacology, Pathophysiology, and Assessment of Biotin Status”, Biotin and Other Interferences in Immunoassays, Elsevier, pp. 17–35, ISBN 978-0-12-816429-7, retrieved 2020-08-27
Avidin is a tetramericbiotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs. Dimeric members of the avidin family are also found in some bacteria. In chicken egg white, avidin makes up approximately 0.05% of total protein (approximately 1800 μg per egg). The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of the avidin-biotin complex is measured to be KD ≈ 10−15 M, making it one of the strongest known non-covalent bonds.
- Helppolainen SH, Nurminen KP, Määttä JA, Halling KK, Slotte JP, Huhtala T, et al. (August 2007). “Rhizavidin from Rhizobium etli: the first natural dimer in the avidin protein family”. The Biochemical Journal. 405 (3): 397–405. doi:10.1042/BJ20070076. PMC 2267316. PMID 17447892.
- Green NM (December 1963). “Avidin. 1. The Use of (14-C)Biotin for Kinetic Studies and for Assay”. The Biochemical Journal. 89 (3
Functional avidin is found in raw egg, but depending on the amount of heat it is exposed to during cooking, the quantity of molecules available for binding biotin can change. The natural function of avidin in eggs is not known, although it has been postulated to be made in the oviduct as a bacterial growth inhibitor, by binding biotin helpful for bacterial growth. As evidence for this, streptavidin, a related protein with equal biotin affinity and a very similar binding site, is made by certain strains of Streptomyces bacteria, and is thought to serve to inhibit the growth of competing bacteria, in the manner of an antibiotic.
- Hendrickson WA, Pähler A, Smith JL, Satow Y, Merritt EA, Phizackerley RP (April 1989). “Crystal structure of core streptavidin determined from multiwavelength anomalous diffraction of synchrotron radiation”. Proceedings of the National Academy of Sciences of the United States of America. 86 (7): 2190–4. Bibcode:1989PNAS…86.2190H. doi:10.1073/pnas.86.7.2190. JSTOR 33443. PMC 286877. PMID 2928324.
A non-glycosylated form of avidin has been isolated from commercially prepared product; however, it is not conclusive as to whether the non-glycosylated form occurs naturally or is a product of the manufacturing process.
- Hiller Y, Gershoni JM, Bayer EA, Wilchek M (November 1987). “Biotin binding to avidin. Oligosaccharide side chain not required for ligand association”. The Biochemical Journal. 248 (1): 167–71. doi:10.1042/bj2480167. PMC 1148514. PMID 3435435.
The biotin-binding properties of avidin were exploited during the development of idrabiotaparinux, a long-acting low molecular weight heparin used in the treatment of venous thrombosis. Due to the long-acting nature of idraparinux, concerns were made about the clinical management of bleeding complications. By adding a biotin moiety to the idraparinux molecule, idrabiotaparinux was formed; its anticoagulant activity in the setting of a bleeding event can be reversed through an intravenous infusion of avidin.
- Büller HR, Gallus AS, Pillion G, Prins MH, Raskob GE (January 2012). “Enoxaparin followed by once-weekly idrabiotaparinux versus enoxaparin plus warfarin for patients with acute symptomatic pulmonary embolism: a randomised, double-blind, double-dummy, non-inferiority trial”. Lancet. 379 (9811): 123–9. doi:10.1016/S0140-6736(11)61505-5. PMID 22130488. S2CID 205964156.
See Heparin: Mechanism of anticoagulant action for a comparison of the mechanism of heparin, low-molecular-weight heparins, fondaparinux and idraparinux.
Uses
Egg white is a fining agent that can be used in the clarification and stabilization of wine. Egg white can also be added to shaken cocktails to create a delicate froth. Some protein powders also use egg whites as a primary source of protein.
Finings are substances that are usually added at or near the completion of the processing of making wine, beer, and various nonalcoholic juice beverages. They are used to remove organic compounds, either to improve clarity or adjust flavor or aroma. The removed compounds may be sulfides, proteins, polyphenols, benzenoids, or copper ions. Unless they form a stable sediment in the final container, the spent finings are usually discarded from the beverage along with the target compounds that they capture.
Substances used as finings include egg whites, blood, milk, isinglass, and Irish moss. These are still used by some producers, but more modern substances have also been introduced and are more widely used, including bentonite, gelatin, casein, carrageenan, alginate, diatomaceous earth, pectinase, pectolyase, PVPP, kieselsol (colloidal silica), copper sulfate, dried albumen (egg whites), hydrated yeast, and activated carbon.[citation needed]
Finings’ actions may be broadly categorized as either electrostatic, adsorbent, ionic, or enzymatic.
The electrostatic types comprise the vast majority; including all but activated carbon, fining yeast, PVPP, copper sulfate, pectinase and pectolase. Their purpose is to selectively remove proteins, tannins (polyphenolics) and coloring particles (melanoidins). They must be used as a batch technique, as opposed to flow-through processing methods such as filters. Their particles each have an electric charge which is attracted to the oppositely charged particles of the colloidal dispersion that they are breaking. The result is that the two substances become bound as a stable complex; their net charge becoming neutral. Thus the agglomeration of a semi-solid follows, which may be separated from the beverage either as a floating or settled mass.
The only adsorbent types of finings in use are activated carbon and specialized fining yeasts. Although activated carbon may be implemented as a flow-through filter, it is also commonly utilized as a batch ingredient, which later must be separated and discarded from the beverage. It can completely/partially remove benzenoid compounds and all classes of polyphenols non-specifically, decolorizing and deodorizing juices and wines. Traditionally, yeast fining has involved the addition of hydrated yeasts used as adsorption agents. Consisting of approximately 30% protein, yeast cell walls have a chemical affinity with wine compounds, such as those that may be polyphenolic or metallic. Indeed, yeast fining is a practical means of removing excess copper ions (greater than 0.5 mg/L) when copper sulfate is used to bind selected volatile sulfur compounds (VSCs).
- Wine/Enology Notes #85, by Bruce Zoecklein, 22 Jan 2004, Virginia Cooperative Extension Service “Enology Notes #85 – Wine Enology Grape Chemistry Lab at Virginia Tech”. Archived from the original on 2006-09-01. Retrieved 2007-05-15.
The ionic finings are copper sulfate and PVPP. When dissolved in aqueous beverages, copper sulfate’s copper ions can chemically bind undesirable sulfides. The resulting complexes must be removed by other finings. The action of PVPP appears to be through the formation of hydrogen bonds between its carbonyl groups and the phenolic hydrogens of the polyphenols. It attracts the low molecular weight polyphenols rather than the condensed tannins and leucanthocyanins that are removed by gelatin.
- Fining & Clarifying Agents, by Terry Rayner Archived 2006-06-16 at the Wayback Machine
The enzymatic finings are pectin and pectinase. They aid in destroying the large polysaccharide molecule named pectin,[clarification needed] which otherwise causes haze in fruit wines and juices. They are among the few finings that are added before juices are fermented.
Unfortunately, beneficial antioxidantflavonoids are removed by some finings. Quercetin is removed from red wines via the finings gelatin, casein, and PVPP to reduce astringent flavors. If other fining methods are used, the quercetin remains in the wine. Similarly the catechin flavonoids are removed by PVPP and other finings that target polyphenolic compounds.
- “Quercetin – Quercetin – Anti-tumor Activity Helps Fight Cancer – Diet and Health.net”. diet-and-health.net. Archived from the original on 2007-09-28.
Flavan-3-ols (sometimes referred to as flavanols, not to be confused with flavonols…these apes…see below )are a subgroup of flavonoids. They are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Flavan-3-ols are structurally diverse and include a range of compounds, such as catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins. They play a part in plant defense and are present in the majority of plants. The single-molecule (monomer) catechin, or isomer epicatechin (see diagram), adds four hydroxyls to flavan-3-ol, making building blocks for concatenated polymers (proanthocyanidins) and higher order polymers (anthocyanidins). Flavan-3-ols possess two chiral carbons, meaning four diastereoisomers occur for each of them. They are distinguished from the yellow, ketone-containing flavonoids such as quercitin and rutin, which are called flavonols. Early use of the term bioflavonoid was imprecisely applied to include the flavanols, which are distinguished by absence of ketone(s). Catechin monomers, dimers, and trimers (oligomers) are colorless. Higher order polymers, anthocyanidins, exhibit deepening reds and become tannins. Catechin and epicatechin are epimers, with (–)-epicatechin and (+)-catechin being the most common optical isomers found in nature. Catechin was first isolated from the plant extract catechu, from which it derives its name. Heating catechin past its point of decomposition releases pyrocatechol (also called catechol), which explains the common origin of the names of these compounds. Epigallocatechin and gallocatechin contain an additional phenolic hydroxyl group when compared to epicatechin and catechin, respectively, similar to the difference in pyrogallol compared to pyrocatechol. Catechin gallates are gallic acid esters of the catechins; an example is epigallocatechin gallate, which is commonly the most abundant catechin in tea. Proanthocyanidins and thearubigins are oligomeric flavan-3-ols. In contrast to many other flavonoids, flavan-3-ols do not generally exist as glycosides in plants. The flavonoids are products from a cinnamoyl-CoA starter unit, with chain extension using three molecules of malonyl-CoA. Reactions are catalyzed by a type III PKS enzyme. These enzyme do not use ACPSs, but instead employ coenzyme A esters and have a single active site to perform the necessary series of reactions, e.g. chain extension, condensation, and cyclization. Chain extension of 4-hydroxycinnamoyl-CoA with three molecules of malonyl-CoA gives initially a polyketide (Figure 1), which can be folded. These allow Claisen-like reactions to occur, generating aromatic rings. Fluorescence-lifetime imaging microscopy (FLIM) can be used to detect flavanols in plant cells.
- Not to be confused with Flavonol. (Flavonols are a class of flavonoids that have the 3-hydroxyflavone backbone (IUPAC name: 3-hydroxy-2-phenylchromen-4-one). Their diversity stems from the different positions of the phenolic –OH groups. They are distinct from flavanols (with “a”) such as catechin, another class of flavonoids, and an unrelated group of metabolically important molecules, the flavins (with “i”), derived from the yellow B vitamin riboflavin. Flavonoids have effects on CYP (P450) activity. Flavonols are inhibitor of CYP2C9 and CYP3A4, which are enzymes that metabolize most drugs in the body. A 2022 study indicated an association between consumption of flavonols (found in food) and a lower rate of decline of cognitive ability, including memory.
- Cermak R, Wolffram S (October 2006). “The potential of flavonoids to influence drug metabolism and pharmacokinetics by local gastrointestinal mechanisms”. Curr. Drug Metab. 7 (7): 729–44. doi:10.2174/138920006778520570. PMID 17073577. Archived from the original on 2012-07-20.
- Si D, Wang Y, Zhou YH, et al. (March 2009). “Mechanism of CYP2C9 inhibition by flavones and flavonols”. Drug Metab. Dispos. 37 (3): 629–34. doi:10.1124/dmd.108.023416. PMID 19074529. S2CID 285706.
- Holland, Thomas Monroe; Agarwal, Puja; Wang, Yamin; Dhana, Klodian; Leurgans, Sue E.; Shea, Kyla; Booth, Sarah L; Rajan, Kumar; Schneider, Julie A.; Barnes, Lisa L. (22 November 2022). “Association of Dietary Intake of Flavonols With Changes in Global Cognition and Several Cognitive Abilities”. Neurology. 100 (7): e694–e702. doi:10.1212/WNL.0000000000201541. PMC 9969915. S2CID 253800625.
- Ullah C, Unsicker SB, Fellenberg C, Constabel CP, Schmidt A, Gershenzon J, Hammerbacher A (December 2017). “Flavan-3-ols Are an Effective Chemical Defense against Rust Infection”. Plant Physiology. 175 (4): 1560–1578. doi:10.1104/pp.17.00842. PMC 5717727. PMID 29070515.
- Schwitters B, Masquelier J (1995). OPC in Practice (3rd ed.). OCLC 45289285.
- Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A (May 2013). “Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases”. Antioxidants & Redox Signaling. 18 (14): 1818–1892. doi:10.1089/ars.2012.4581. PMC 3619154. PMID 22794138.
- Dewick PM (2009). Medicinal Natural Products: a biosynthetic approach. John Wiley & Sons. p. 168. ISBN 978-0-471-49641-0.
- Winkel-Shirley B (June 2001). “Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology”. Plant Physiology. 126 (2): 485–493. doi:10.1104/pp.126.2.485. PMC 1540115. PMID 11402179.
- Mueller-Harvey I, Feucht W, Polster J, Trnková L, Burgos P, Parker AW, Botchway SW (March 2012). “Two-photon excitation with pico-second fluorescence lifetime imaging to detect nuclear association of flavanols”. Analytica Chimica Acta. 719: 68–75. doi:10.1016/j.aca.2011.12.068. PMID 22340533. S2CID 24094780.
- “Database on polyphenol content in foods, v. 3.6”. Phenol Explorer. 2016.
See also: Polyphenols in tea, Polyphenols in wine, and Cocoa bean § Phytochemicals and research
Flavan-3-ols are abundant in teas derived from the tea plant Camellia sinensis, as well as in some cocoas (made from the seeds of Theobroma cacao), although the content is affected considerably by processing, especially in chocolate. Flavan-3-ols are also present in the human diet in fruits, in particular pome fruits, berries, vegetables, and wine. Their content in food is variable and affected by various factors, such as cultivar, processing, and preparation. The bioavailability of flavan-3-ols depends on the food matrix, type of compound and their stereochemical configuration. While monomeric flavan-3-ols are readily taken up, oligomeric forms are not absorbed. Most data for human metabolism of flavan-3-ols are available for monomeric compounds, especially epiatechin. These compounds are taken up and metabolized upon uptake in the jejunum, mainly by O-methylation and glucuronidation, and then further metabolized by the liver. The colonic microbiome has also an important role in the metabolism of flavan-3-ols and they are catabolized to smaller compounds such as 5-(3′/4′-dihydroxyphenyl)-γ-valerolactones and hippuric acid. Only flavan-3-ols with an intact (epi)catechin moiety can be metabolized into 5-(3′/4′-dihydroxyphenyl)-γ-valerolactones (image in Gallery). As catechins in green tea extract can be hepatotoxic, Health Canada and EFSA have advised for caution, recommending intake should not exceed 800 mg per day.
See also: Cocoa bean § Phytochemicals and research
- Hammerstone JF, Lazarus SA, Schmitz HH (August 2000). “Procyanidin content and variation in some commonly consumed foods”. The Journal of Nutrition. 130 (8S Suppl): 2086S–2092S. doi:10.1093/jn/130.8.2086S. PMID 10917927.
- Payne MJ, Hurst WJ, Miller KB, Rank C, Stuart DA (October 2010). “Impact of fermentation, drying, roasting, and Dutch processing on epicatechin and catechin content of cacao beans and cocoa ingredients”. Journal of Agricultural and Food Chemistry. 58 (19): 10518–10527. doi:10.1021/jf102391q. PMID 20843086.
- Mabrym H, Harborne JB, Mabry TJ (1975). The Flavonoids. London: Chapman and Hall. ISBN 978-0-412-11960-6.
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (May 2004). “Polyphenols: food sources and bioavailability”. The American Journal of Clinical Nutrition. 79 (5): 727–747. doi:10.1093/ajcn/79.5.727. PMID 15113710.
- Del Río D, Rodríguez Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A (May 2013). “Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases”. Antioxidants & Redox Signaling. 18 (14): 1818–1892. doi:10.1089/ars.2012.4581. PMC 3619154. PMID 22794138.
- Rodríguez Mateos A, Weber T, Skene SS, Ottaviani JI, Crozier A, Kelm M, et al. (December 2018). “Assessing the respective contributions of dietary flavanol monomers and procyanidins in mediating cardiovascular effects in humans: randomized, controlled, double-masked intervention trial”. The American Journal of Clinical Nutrition. 108 (6): 1229–1237. doi:10.1093/ajcn/nqy229. PMC 6290365. PMID 30358831.
- Actis-Goretta L, Lévèques A, Rein M, Teml A, Schäfer C, Hofmann U, et al. (October 2013). “Intestinal absorption, metabolism, and excretion of (−)-epicatechin in healthy humans assessed by using an intestinal perfusion technique”. The American Journal of Clinical Nutrition. 98 (4): 924–933. doi:10.3945/ajcn.113.065789. PMID 23864538.
- Kuhnle G, Spencer JP, Schroeter H, Shenoy B, Debnam ES, Srai SK, et al. (October 2000). “Epicatechin and catechin are O-methylated and glucuronidated in the small intestine”. Biochemical and Biophysical Research Communications. 277 (2): 507–512. doi:10.1006/bbrc.2000.3701. PMID 11032751.
- Das NP (December 1971). “Studies on flavonoid metabolism. Absorption and metabolism of (+)-catechin in man”. Biochemical Pharmacology. 20 (12): 3435–3445. doi:10.1016/0006-2952(71)90449-7. PMID 5132890.
- Ottaviani JI, Borges G, Momma TY, et al. (July 2016). “The metabolome of [2-14C](−)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives”. Scientific Reports. 6 (1): 29034. Bibcode:2016NatSR…629034O. doi:10.1038/srep29034. PMC 4929566. PMID 27363516.
- Ottaviani JI, Fong R, Kimball J, Ensunsa JL, Britten A, Lucarelli D, et al. (June 2018). “Evaluation at scale of microbiome-derived metabolites as biomarker of flavan-3-ol intake in epidemiological studies”. Scientific Reports. 8 (1): 9859. Bibcode:2018NatSR…8.9859O. doi:10.1038/s41598-018-28333-w. PMC 6026136. PMID 29959422.
- Health Canada (12 December 2017). “Summary Safety Review – Green tea extract-containing natural health products – Assessing the potential risk of liver injury (hepatotoxicity)”. Health Canada, Government of Canada. Retrieved 2022-05-06.
- Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, et al. (April 2018). “Scientific opinion on the safety of green tea catechins”. EFSA Journal. 16 (4): e05239. doi:10.2903/j.efsa.2018.5239. PMC 7009618. PMID 32625874.
Catechins are metabolised upon uptake from the gastrointestinal tract, in particular the jejunum, and in the liver, resulting in so-called structurally related epicatechin metabolites (SREM). The main metabolic pathways for SREMs are glucuronidation, sulfation and methylation of the catechol group by catechol-O-methyl transferase, with only small amounts detected in plasma. The majority of dietary catechins are however metabolised by the colonic microbiome to gamma-valerolactones and hippuric acids which undergo further biotransformation, glucuronidation, sulfation and methylation in the liver. The stereochemical configuration of catechins has a strong impact on their uptake and metabolism as uptake is highest for (−)-epicatechin and lowest for (−)-catechin.
- Ottaviani JI, Borges G, Momma TY, Spencer JP, Keen CL, Crozier A, Schroeter H (July 2016). “The metabolome of [2-14C](−)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives”. Scientific Reports. 6: 29034. Bibcode:2016NatSR…629034O. doi:10.1038/srep29034. PMC 4929566. PMID 27363516.
- Actis-Goretta L, Lévèques A, Rein M, Teml A, Schäfer C, Hofmann U, et al. (October 2013). “Intestinal absorption, metabolism, and excretion of (−)-epicatechin in healthy humans assessed by using an intestinal perfusion technique”. The American Journal of Clinical Nutrition. 98 (4): 924–933. doi:10.3945/ajcn.113.065789. PMID 23864538.
- Ottaviani JI, Momma TY, Kuhnle GK, Keen CL, Schroeter H (April 2012). “Structurally related (−)-epicatechin metabolites in humans: assessment using de novo chemically synthesized authentic standards”. Free Radical Biology & Medicine. 52 (8): 1403–1412. doi:10.1016/j.freeradbiomed.2011.12.010. PMID 22240152.
- “Flavonoids”. Linus Pauling Institute, Oregon State University, Corvallis. 2016. Retrieved 24 July 2016.
- Ottaviani JI, Momma TY, Heiss C, Kwik-Uribe C, Schroeter H, Keen CL (January 2011). “The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo”. Free Radical Biology & Medicine. 50 (2): 237–244. doi:10.1016/j.freeradbiomed.2010.11.005. PMID 21074608.
Biotransformation
Biotransformation of (+)-catechin into taxifolin by a two-step oxidation can be achieved by Burkholderia sp.[35]
(+)-Catechin and (−)-epicatechin are transformed by the endophytic filamentous fungus Diaporthe sp. into the 3,4-cis-dihydroxyflavan derivatives, (+)-(2R,3S,4S)-3,4,5,7,3′,4′-hexahydroxyflavan (leucocyanidin) and (−)-(2R,3R,4R)-3,4,5,7,3′,4′-hexahydroxyflavan, respectively, whereas (−)-catechin and (+)-epicatechin with a (2S)-phenyl group resisted the biooxidation.[36]
Leucoanthocyanidin reductase (LAR) uses (2R,3S)-catechin, NADP+ and H2O to produce 2,3-trans-3,4-cis-leucocyanidin, NADPH, and H+. Its gene expression has been studied in developing grape berries and grapevine leaves.[37]
Glycosides
- (2R,3S)-Catechin-7-O-β-D-glucopyranoside can be isolated from barley (Hordeum vulgare L.) and malt.[38]
- Epigeoside (catechin-3-O-α-L-rhamnopyranosyl-(1–4)-β-D-glucopyranosyl-(1–6)-β-D-glucopyranoside) can be isolated from the rhizomes of Epigynum auritum.[39]
Polyvinylpolypyrrolidone (polyvinyl polypyrrolidone, PVPP, crospovidone, crospolividone, or E1202) is a highly cross-linked modification of polyvinylpyrrolidone (PVP). The cross-linked form of PVP is used as a disintegrant (see also excipients) in pharmaceutical tablets. PVPP is a highly cross-linked version of PVP, making it insoluble in water, though it still absorbs water and swells very rapidly generating a swelling force. This property makes it useful as a disintegrant in tablets. PVPP can be used as a drug, taken as a tablet or suspension to absorb compounds (so-called endotoxins) that cause diarrhea. (Cf. bone char, charcoal.) It is also used as a fining to extract impurities (via agglomeration followed by filtration). It is used in winemaking. Using the same principle it is used to remove polyphenols in beer production and thus clear beers with stable foam are produced. One such commercial product is called Polyclar. PVPP forms bonds similar to peptidic bonds in protein (especially, like proline residues) and that is why it can precipitate tannins the same way as proteins do. PVPP has E number code E1202 and is used as a stabiliser.
- “Povidones, Copovidones, and Crospovidones for Pharmaceutical Products”. BASF Pharma. Retrieved 2022-06-11.
- Microsoft Word – G0294.doc[dead link]
- “A Novel Stabilization of Beer with Polyclar Brewbrite. Mustafa Rehmanji, Chandra Gopal, and Andrew Mola, MBAA TQ vol. 39, no. 1, 2002, pp. 24–28” (PDF). Archived from the original (PDF) on 2015-09-06. Retrieved 2017-05-19.
Local links concerning Quercetin
See also
Main articles: Vegetarianism and wine and Vegetarianism and beer
Since some finings are animal products and others are not, it can be difficult for consumers to find out whether a particular wine or beer is vegan, vegetarian, or neither, unless the producer or seller chooses to label it as such. The website Barnivore maintains an international database of wines and beers, classifying each as “Vegan Friendly” or “Not Vegan Friendly”
- “About Vegan Wine | What is it and why isn’t all wine vegan?”. Vegan Wine Box. Retrieved 2020-09-11.
- Smith, Stacey (16 January 2018). “10 best vegan wines”. The Independent. Retrieved 17 March 2021.
See also
- Clarifying agent
- Clarification and stabilization of wine#Fining
- Beer Clarifying Agent Archived 2018-09-21 at the Wayback Machine & Wine Clarifying Agent Archived 2018-09-21 at the Wayback Machine
The albumen from egg white was used as a binding agent in early photography during an 1855-90 period; such prints were called albumen prints.
In the 1750s, egg whites were believed to prevent swelling, and were used for that purpose. To help soothe areas of skin that were afflicted, egg white mixed with Armenian bole could help restore the fibers. Egg whites are also used in bookbinding during the gilding process, where it is referred to as ‘glaire’, and to give a book cover shine.
Old French glaire, from Vulgar Latin *clāria, from Latin clarus (“clear”). plural glaires
“Leathers require a medium for permanently fixing down the gold leaf. This substance is called glaire and is made up by adding 2 parts of dry albumen 2 parts of vinegar with 5 parts of water. Soak over night beat up well next morning allow to settle then strain off the clear part of the resulting liquid and bottle for use as required in smaller quantities. When glairing in grained leathers such as Morocco a froth has a tendency to form this can be dissipated by adding a spot or two of milk to an eggcup full of glaire. The raw white of an egg is another form of pure albumen but the dried article which is sold per pound is more economical and handy for use. To clean off superfluous gold leaf rubber reduced to a tacky consistency is used. To make this pure raw Para rubber should be cut into small pieces and left in paraffin all night working it all together again as one would a piece of putty In the course of time this becomes loaded with the gold leaf and can be sold for recovering the gold”
J. Leonard Monk, W. F. Lawrence, A Text Book of Stationery Binding: A Treatise on the Whole Art of Forwarding and Finishing Stationery Books : Including Chapters on Ruling, Marbling, Leathers, and Papers, Raithby, Lawrence, 1912 (Available at Google Books)

- de Vandenesse, Urbain (2011). “Egg White”. Translated by Abigail Wendler Bainbridge. The Encyclopedia of Diderot & d’Alembert Collaborative Translation Project. Retrieved 31 March 2015.
Translation of “Blanc d’oeuf,” Encyclopédie ou Dictionnaire raisonné des sciences, des arts et des métiers, vol. 2. Paris, 1752
- What is Para rubber used for? The seeds contain Para rubber seed oil, a semi-drying pale yellow oil used for illumination, soap making, paint, varnish, and as a treatment against house flies and lice. The plant is often intercropped with coffee or cocoa. Tapping the trunk yields latex used in wide range of applications. uh huh…i mean holy fucking shit. this is too much.
- Hevea brasiliensis Para Rubber Tree, Brazilian … – PFAF.org
- Natural rubber is also called para rubber.
- There is a tree called Para rubber
- A Para rubber tree (or simply, rubber tree) is the tree which naturally produces rubber. It is native to tropical areas from South America, in the Amazon (Brazil), but was spread by European farmers to the Far East. Para Rubber trees belong to the Euphorbiaceae family. In the wild they may reach heights of 100 to 125 ft (30-38 m) with large cylindrical trunks with or without buttresses. Crop trees reach a width of about 20 in. (50 cm), usually with a short bole, and with a sloped taper. Most rubber plantations are in South Asia and Southeast Asia. When trees reach 5-6 years old, they are harvested. Their trunks are cut just deep enough to tap the vessels without harming the tree’s growth, and the sap is collected in small buckets. This process is known as rubber tapping. Older trees produce more latex, but they stop producing it after 30-40 years. It was first collected in para district of brazil. Sri henry Wickham brought the seeds of para rubber from Brazil to India and Sri Lanka through the kew botanical garden in 1876. At first, the plantation was done in Malayasia and Sri Lanka and later in Java, Sumatra, India and parts of Indonesia.
Armenian bole, also known as bolus armenus or bole armoniac, is an earthy clay, usually red, native to Armenia but also found in other places. The term Armenian was later referred to a specific quality of the clay. Originally used in medication, it has also been used as a pigment, as a poliment or base for gilding, and for other uses. It is red due to the presence of iron oxide; the clay also contains hydrous silicates of aluminum and possibly magnesium.
Uses

Historically, the term bolu or bolus was used only for medicinal earths and Armenian bole was used as an astringent, prescribed against diarrhea, dysentery, and bleeding. References to Armenian bole were made by Theophrastus, Dioscorides (c. 41–90 AD) and Pliny the Elder (23–79 AD). Externally, it was used in strengthening plasters, against dislocations of the joints. Physicians sometimes also called it Rubrica Synopica, from the city of Synope, where it is supposed to be found. Use for internal medicine may have side effects as the minerals often include heavy metals such as arsenic, cadmium, lead and zinc that can cause toxicity.
- Hosseinkhani A; Montaseri H; Mohagheghzadeh A; Seradj H; Sodaifi M. (2014). “Armenian bole: a historical medicinal clay”. Pharmaceutical Historian. 44 (4): 98–100. PMID 25966606.
- Gomes, Celso de Sousa Figueiredo (2018). “Healing and edible clays: a review of basic concepts, benefits and risks”. Environmental Geochemistry and Health. 40 (5): 1739–1765. doi:10.1007/s10653-016-9903-4. ISSN 0269-4042. PMID 28150053.
- Hosamo, Ammar; Zarshenas, Mohammad Mehdi; Mehdizadeh, Alireza; Zomorodian, Kamiar; Khani, Ayda Hossein (2016). “The Effect of Traditional Treatments on Heavy Metal Toxicity of Armenian Bole”. Iranian Journal of Medical Sciences. 41 (3 Suppl): S65. ISSN 0253-0716. PMC 5103575. PMID 27840531.
In the nineteenth century, it was incorporated into non-soluble tooth powder. These types of powders would get stuck between the gums and the teeth and leave an unsightly discoloration. As a result, they were coloured red using Armenian bole to disguise the buildup around the teeth.
- Foulk, Martha E.; Pickering, Elizabeth (1935). “A History of Dentrifices”. Journal of the American Pharmaceutical Association. 24 (11): 975.
It is also used in bookbinding for coloring, or applied to the edges during gilding, as a base for the gold leaf and to give the binding a greater depth and luster. In pottery, it is used as a red pigment for the İznik pottery of Turkey. Finally, it has also been used in the waterproofing of windmill sails. A popular mixture was: 10 liters of water, combined with 0.75 liter linseed oil, 0.75 liter grease, and 1 kg of bolus.
- de Vandenesse, Urbain (2011). “Egg White”. Translated by Abigail Wendler Bainbridge. The Encyclopedia of Diderot & d’Alembert Collaborative Translation Project. Retrieved 31 March 2015.
Translation of “Blanc d’oeuf,” Encyclopédie ou Dictionnaire raisonné des sciences, des arts et des métiers, vol. 2. Paris, 1752
- Barata, C.; Rocha, F.; Cruz, A.J.; Andrejkovičová, S.; Reguer, S. (2015). “Synchrotron X-ray diffraction of bole layers from Portuguese gilded baroque retables”. Applied Clay Science. 116–117: 39–45. doi:10.1016/j.clay.2015.08.012.
- Hradil, David; Hradilová, Janka; Bezdička, Petr; Serendan, Cristina (2017). “Late Gothic/early Renaissance gilding technology and the traditional poliment material “Armenian bole”: Truly red clay, or rather bauxite?”. Applied Clay Science. 135: 271–281. doi:10.1016/j.clay.2016.10.004.
- Werken met molens by Werkgroep West-Vlaamse Molens v.z.w.
- Bolus used for windmill sail
See also
- Levant bole, a similar clay, often used in place of Armenian bole
- Levant bole is an earthy clay brought from the Levant, and historically used in medicine for the same purposes as Armenian bole. It was indeed so similar to Armenian bole that some believed them both to be the same, or at least mixtures of each other. Levant bole was used in several compositions, particularly diascodium, to give it color.
- Chambers discusses two other similar boles:
- Lemnian or Terra Lemia from the island of Lemnos, also called Sigillata
- Samnian or Terra Samia from the island of Samos
- This article incorporates text from a publication now in the public domain: Chambers, Ephraim, ed. (1728). “Bole”. Cyclopædia, or an Universal Dictionary of Arts and Sciences (1st ed.). James and John Knapton, et al.

Powdered Eggs
A powdered egg is a fully dehydrated egg. Most powdered eggs are made using spray drying in the same way that powdered milk is made.[further explanation needed] The major advantages of powdered eggs over fresh eggs are the reduced weight per volume of whole egg equivalent and the shelf life. Other advantages include smaller usage of storage space, and lack of need for refrigeration. Powdered eggs can be used without rehydration when baking, and can be rehydrated to make dishes such as scrambled eggs and omelettes.
History
Dehydrated eggs advertisements appeared in the late 1890s in the United States. Powdered eggs appear in literature as a staple of camp cooking at least as early as 1912.
- unidentified, English (1898-03-05), English: Based in St. Louis, Charles Fred LaMont’s company produced egg substitutes and partly targeted miners of the Alaska Gold Rush. In 1898, the manufacturers shipped over 100,000 pounds of Crystallized Eggs to South African miners., retrieved 2022-02-28
- Along the Mohawk trail; or, Boy scouts on Lake Champlain, Percy Keese Fitzhugh, Grosset & Dunlap, 1912, p. 219.
Powdered eggs were used in the United Kingdom during World War II for rationing. Powdered eggs are also known as dried eggs, and colloquially during the period of rationing in the UK, as Ersatz eggs.

The modern method of manufacturing powdered eggs was developed in the 1930s by Albert Grant and Co. of the Mile End Road, London. The cake manufacturer was importing liquid egg from China and one of his staff realised that this was 75% water. An experimental freeze-drying plant was built and tried. Then a factory was set up in Singapore to process Chinese egg.[clarification needed] As war approached, Grant transferred his dried egg facility to Argentina. The British Government lifted the patent[which?] during the war and many other suppliers came into the market, notably in the United States. Early importers to the United States included Vic Henningsen Sr. and others in the United Kingdom.
Quality
Powdered eggs have a storage life of 5 to 10 years when stored without oxygen in a cool storage environment.
- “Powdered Eggs”. USA Emergency Supply. Retrieved 27 February 2018.
The process of spray-drying eggs so as to make powdered eggs oxidizes the cholesterol, which has been shown to be helpful at reducing aortic atherosclerosis in animal trials.
- Griminger, P; Fisher, H (1986). “The effect of dried and fresh eggs on plasma cholesterol and atherosclerosis in chickens”. Poultry Science. 65 (5): 979–82. doi:10.3382/ps.0650979. PMID 3725728.
Spray drying is a method of forming a dry powder from a liquid or slurry by rapidly drying with a hot gas. This is the preferred method of drying of many thermally-sensitive materials such as foods and pharmaceuticals, or materials which may require extremely consistent, fine particle size. Air is the heated drying medium; however, if the liquid is a flammable solvent such as ethanol or the product is oxygen-sensitive then nitrogen is used.
- Campbell, Heather R.; Alsharif, Fahd M.; Marsac, Patrick J.; Lodder, Robert A. (2020). “The Development of a Novel Pharmaceutical Formulation of D-Tagatose for Spray-Drying”. Journal of Pharmaceutical Innovation: 1–13. doi:10.1007/s12247-020-09507-4.
- A. S. Mujumdar (2007). Handbook of industrial drying. CRC Press. p. 710. ISBN 978-1-57444-668-5.
All spray dryers use some type of atomizer or spray nozzle to disperse the liquid or slurry into a controlled drop size spray. The most common of these are rotary disk and single-fluid high pressure swirl nozzles. Atomizer wheels are known to provide broader particle size distribution, but both methods allow for consistent distribution of particle size. Alternatively, for some applications two-fluid or ultrasonic nozzles are used. Depending on the process requirements, drop sizes from 10 to 500 μm can be achieved with the appropriate choices. The most common applications are in the 100 to 200 μm diameter range. The dry powder is often free-flowing.
- “Contract Spray Dryer & Spray Drying Services | Elan”.
- Walter R. Niessen (2002). Combustion and incineration processes. CRC Press. p. 588. ISBN 978-0-8247-0629-6.
The most common type of spray dryers are called single effect. There is a single source of drying air at the top of the chamber (see n°4 on the diagram). In most cases the air is blown in the same direction as the sprayed liquid (co-current). A fine powder is produced, but it can have poor flow and produce much dust. To overcome the dust and poor flow of the powder, a new generation of spray dryers called multiple effect spray dryers have been produced. Instead of drying the liquid in one stage, drying is done through two steps: the first at the top (as per single effect) and the second with an integrated static bed at the bottom of the chamber. The bed provides a humid environment which causes smaller particles to clump, producing more uniform particle sizes, usually within the range of 100 to 300 μm. These powders are free-flowing due to the larger particle size.[citation needed]
The fine powders generated by the first stage drying can be recycled in continuous flow either at the top of the chamber (around the sprayed liquid) or at the bottom, inside the integrated fluidized bed. The drying of the powder can be finalized on an external vibrating fluidized bed.
The hot drying gas can be passed in as a co-current, same direction as sprayed liquid atomizer, or counter-current, where the hot air flows against the flow from the atomizer. With co-current flow, particles spend less time in the system and the particle separator (typically a cyclone device). With counter-current flow, particles spend more time in the system and is usually paired with a fluidized bed system. Co-current flow generally allows the system to operate more efficiently.
Alternatives to spray dryers are:
- Freeze dryer: a more-expensive batch process for products that degrade in spray drying. Dry product is not free-flowing.
- Drum dryer: a less-expensive continuous process for low-value products; creates flakes instead of free-flowing powder.
- Pulse combustion dryer: A less-expensive continuous process that can handle higher viscosities and solids loading than a spray dryer, and sometimes yields a freeze-dry quality powder that is free-flowing.
- Charles Onwulata (2005). Encapsulated and powdered foods. CRC Press. p. 268. ISBN 978-0-8247-5327-6. p. 66
History
The spray drying technique was first described in 1860 with the first spray dryer instrument patented by Samuel Percy in 1872.[citation needed] With time, the spray drying method grew in popularity, at first mainly for milk production in the 1920s and during World War II, when there was a need to reduce the weight and volume of food and other materials. In the second half of the 20th century, commercialization of spray dryers increased, as did the number of spray drying applications.
Spray dryer
A spray dryer takes a liquid stream and separates the solute or suspension as a solid and the solvent into a vapor. The solid is usually collected in a drum or cyclone. The liquid input stream is sprayed through a nozzle into a hot vapor stream and vaporized. Solids form as moisture quickly leaves the droplets. A nozzle is usually used to make the droplets as small as possible, maximizing surface area hence heat transfer and the rate of water vaporization. Droplet sizes can range from 20 to 180 μm depending on the nozzle. There are two main types of nozzles: high pressure single fluid nozzle (50 to 300 bars) and two-fluid nozzles: one fluid is the liquid to dry and the second is compressed gas (generally air at 1 to 7 bars).
- Walter R. Niessen (2002). Combustion and incineration processes. CRC Press. p. 588. ISBN 978-0-8247-0629-6.
Spray dryers can dry a product very quickly compared to other methods of drying. They also turn a solution (or slurry) into a dried powder in a single step, which simplifies the process and improves profit margins.
In pharmaceutical manufacturing, spray drying is employed to manufacture Amorphous Solid Dispersions, by uniformly dispersing Active Pharmaceutical Ingredients into a polymer matrix. This state will put the active compounds (drug) in a higher state of energy which in turn facilitates diffusion of drug species in patient body.
- Poozesh, Sadegh; Lu, Kun; Marsac, Patrick J. (July 2018). “On the particle formation in spray drying process for bio-pharmaceutical applications: Interrogating a new model via computational fluid dynamics”. International Journal of Heat and Mass Transfer. 122: 863–876. doi:10.1016/j.ijheatmasstransfer.2018.02.043.
Micro-encapsulation
Spray drying often is used as an encapsulation technique by the food and other industries. A substance to be encapsulated (the load) and an amphipathic carrier (usually some sort of modified starch) are homogenized as a suspension in water (the slurry). The slurry is then fed into a spray drier, usually a tower heated to temperatures above the boiling point of water.
As the slurry enters the tower, it is atomized. Partly because of the high surface tension of water and partly because of the hydrophobic/hydrophilic interactions between the amphipathic carrier, the water, and the load, the atomized slurry forms micelles. The small size of the drops (averaging 100 micrometers in diameter) results in a relatively large surface area which dries quickly. As the water dries, the carrier forms a hardened shell around the load.
- Ajay Kumar (2009). Bioseparation Engineering. I. K. International. p. 179. ISBN 978-93-8002-608-4.
Load loss is usually a function of molecular weight. That is, lighter molecules tend to boil off in larger quantities at the processing temperatures. Loss is minimized industrially by spraying into taller towers. A larger volume of air has a lower average humidity as the process proceeds. By the osmosis principle, water will be encouraged by its difference in fugacities in the vapor and liquid phases to leave the micelles and enter the air. Therefore, the same percentage of water can be dried out of the particles at lower temperatures if larger towers are used. Alternatively, the slurry can be sprayed into a partial vacuum. Since the boiling point of a solvent is the temperature at which the vapor pressure of the solvent is equal to the ambient pressure, reducing pressure in the tower has the effect of lowering the boiling point of the solvent.
The application of the spray drying encapsulation technique is to prepare “dehydrated” powders of substances which do not have any water to dehydrate. For example, instant drink mixes are spray dries of the various chemicals which make up the beverage. The technique was once used to remove water from food products. One example is the preparation of dehydrated milk. Because the milk was not being encapsulated and because spray drying causes thermal degradation, milk dehydration and similar processes have been replaced by other dehydration techniques. Skim milk powders are still widely produced using spray drying technology, typically at high solids concentration for maximum drying efficiency. Thermal degradation of products can be overcome by using lower operating temperatures and larger chamber sizes for increased residence times.
- Charles Onwulata (2005). Encapsulated and powdered foods. CRC Press. p. 268. ISBN 978-0-8247-5327-6. pp.389–430
Recent research is now suggesting that the use of spray-drying techniques may be an alternative method for crystallization of amorphous powders during the drying process since the temperature effects on the amorphous powders may be significant depending on drying residence times.
- Charles Onwulata (2005). Encapsulated and powdered foods. CRC Press. p. 268. ISBN 978-0-8247-5327-6. p.268
- Chiou, D.; Langrish, T. A. G. (2007). “Crystallization of Amorphous Components in Spray-Dried Powders”. Drying Technology. 25 (9): 1427–1435. doi:10.1080/07373930701536718.
Designing particle shape and size
The spray drying process contains a variety of input parameters that can alter the shape and size of yielded particles.
Common input parameters:
- Solution Concentration
- Drying Gas Flow
- Inlet Temperature
- Spraying Gas Flow
- Feed Rate
From the following input parameters comes a series of pathways a particle can take towards its yielded shape and size. Certain parameters like spraying gas flow, feed rate, and the solution concentration heavily influence the yielded particle size, whereas the inlet temperature plays a significant role into the shape of the particle at the end. Particle size has a great correlation with the original size of the solution droplet from the atomizer, so the greatest way to control particle size can be done by heavily saturating the solution and making the initial droplet larger or smaller. Once the initial droplet enters the drying chamber, the droplet can continue to crust formation, or no particle will be formed. From the crust formation, the temperature of the drying process and duration of the particle in the drying process can lead the particle toward a dry shell or a deformed particle. The dry shell can proceed into a solid particle or a shattered particle. The crust formation can also forgo the dry shell or deformed particle if the drying conditions are not correct and undergo an internal bubble nucleation with another series of pathways.
The current understanding of the drying conditions varies between different spray drying configurations and solution contents, but more research is being completed into the determination of what drives each particle shape pathways as future applications in pharmaceutical and industrial areas require better control over specific particle shapes and sizes of their products.
Spray drying applications
Food: milk powder, coffee, tea, eggs, cereal, spices, flavorings, blood, starch and starch derivatives, vitamins, enzymes, stevia, nutracutical, colourings, animal feed, etc.
- Heuzé V.; Tran G. (2016) [Last updated on March 31, 2016, 10:31]. “Blood meal”. Feedipedia. a programme by INRA, CIRAD, AFZ and FAO.
Pharmaceutical: antibiotics, medical ingredients, additives.
- Ting, Jeffrey M.; Porter, William W.; Mecca, Jodi M.; Bates, Frank S.; Reineke, Theresa M. (2018-01-10). “Advances in Polymer Design for Enhancing Oral Drug Solubility and Delivery”. Bioconjugate Chemistry. 29 (4): 939–952. doi:10.1021/acs.bioconjchem.7b00646. ISSN 1043-1802. PMID 29319295.
- Ricarte, Ralm G.; Van Zee, Nicholas J.; Li, Ziang; Johnson, Lindsay M.; Lodge, Timothy P.; Hillmyer, Marc A. (2019-09-05). “Recent Advances in Understanding the Micro- and Nanoscale Phenomena of Amorphous Solid Dispersions”. Molecular Pharmaceutics. 16 (10): 4089–4103. doi:10.1021/acs.molpharmaceut.9b00601. ISSN 1543-8384. PMID 31487183.
Industrial: paint pigments, ceramic materials, catalyst supports, microalgae.
See also
- Food powder
- List of dried foods
- Egg yolk
- Egg white substitutes
- Albumen print
- Haugh unit, a unit of measure for egg albumen
- The Haugh unit is a measure of egg protein quality based on the height of its egg white (albumen). The test was introduced by Raymond Haugh in 1937 and is an important industry measure of egg quality next to other measures such as shell thickness and strength.[citation needed] An egg is weighed, then broken onto a flat surface (breakout method), and a micrometer used to determine the height of the thick albumen (egg white) that immediately surrounds the yolk. The height, correlated with the weight, determines the Haugh unit, or HU, rating. The higher the number, the better the quality of the egg (fresher, higher quality eggs have thicker whites). Although the measurement determines the protein content and freshness of the egg, it does not measure other important nutrient contents such as the micronutrient or vitamins present in the egg.
- Jeffrey Kluger (July 8, 2010). “Organic Eggs: More Expensive, but No Healthier”. Time magazine. Archived from the original on July 9, 2010. Retrieved 2010-07-11.
- The Haugh unit is a measure of egg protein quality based on the height of its egg white (albumen). The test was introduced by Raymond Haugh in 1937 and is an important industry measure of egg quality next to other measures such as shell thickness and strength.[citation needed] An egg is weighed, then broken onto a flat surface (breakout method), and a micrometer used to determine the height of the thick albumen (egg white) that immediately surrounds the yolk. The height, correlated with the weight, determines the Haugh unit, or HU, rating. The higher the number, the better the quality of the egg (fresher, higher quality eggs have thicker whites). Although the measurement determines the protein content and freshness of the egg, it does not measure other important nutrient contents such as the micronutrient or vitamins present in the egg.
- Meringue, a dessert or dessert ingredient made from egg white
- Protein quality
References
Wikimedia Commons has media related to Albumen prints.
- Blanquart-Evrard, Louis-Désiré (1869). La photographie, ses origines, ses progrès, ses transformations (in French). Lille, France: L. Danel.
- Newhall, Beaumont (April 1955). “60,000 Eggs A Day” (PDF). Image, Journal of Photography of George Eastman House. Rochester, N.Y.: International Museum of Photography at George Eastman House Inc. IV (4): 25–26. Archived from the original (PDF) on 4 March 2016. Retrieved 20 July 2014.
- Welling, William. Photography in America (1978 & 1987)
- Hosseinkhani A; Montaseri H; Mohagheghzadeh A; Seradj H; Sodaifi M. (2014). “Armenian bole: a historical medicinal clay”. Pharmaceutical Historian. 44 (4): 98–100. PMID 25966606.
- Gomes, Celso de Sousa Figueiredo (2018). “Healing and edible clays: a review of basic concepts, benefits and risks”. Environmental Geochemistry and Health. 40 (5): 1739–1765. doi:10.1007/s10653-016-9903-4. ISSN 0269-4042. PMID 28150053.
- Hosamo, Ammar; Zarshenas, Mohammad Mehdi; Mehdizadeh, Alireza; Zomorodian, Kamiar; Khani, Ayda Hossein (2016). “The Effect of Traditional Treatments on Heavy Metal Toxicity of Armenian Bole”. Iranian Journal of Medical Sciences. 41 (3 Suppl): S65. ISSN 0253-0716. PMC 5103575. PMID 27840531.
- Foulk, Martha E.; Pickering, Elizabeth (1935). “A History of Dentrifices”. Journal of the American Pharmaceutical Association. 24 (11): 975.
- Barata, C.; Rocha, F.; Cruz, A.J.; Andrejkovičová, S.; Reguer, S. (2015). “Synchrotron X-ray diffraction of bole layers from Portuguese gilded baroque retables”. Applied Clay Science. 116–117: 39–45. doi:10.1016/j.clay.2015.08.012.
- Hradil, David; Hradilová, Janka; Bezdička, Petr; Serendan, Cristina (2017). “Late Gothic/early Renaissance gilding technology and the traditional poliment material “Armenian bole”: Truly red clay, or rather bauxite?”. Applied Clay Science. 135: 271–281. doi:10.1016/j.clay.2016.10.004.
- Werken met molens by Werkgroep West-Vlaamse Molens v.z.w.
- Bolus used for windmill sail
- Ornithology, Volume 1994 By Frank B. Gill p. 361
- James, John M.; Zeiger, Robert S.; Lester, Mitchell R.; Fasano, Mary Beth; Gern, James E.; Mansfield, Lyndon E.; Schwartz, Howard J.; Sampson, Hugh A.; Windom, Hugh H.; Machtinger, Steven B.; Lensing, Shelly (1998). “Safe administration of influenza vaccine to patients with egg allergy”. The Journal of Pediatrics. 133 (5): 624–8. doi:10.1016/S0022-3476(98)70101-5. PMID 9821418.
- McGee, Harold. On Food and Cooking: The Science and Lore of the Kitchen. New York: Scribner, 2004, edited by Vinay.[page needed]
- Exploratorium
- Takehiko Yamamoto, Mujo Kim (1996-12-13), Hen eggs, ISBN 9780849340055
- “Science of Cooking: Ask the Inquisitive Cooks!”. exploratorium.edu.
- McGee, Harold J.; Long, Sharon R.; Briggs, Winslow R. (1984). “Why whip egg whites in copper bowls?”. Nature. 308 (5960): 667–8. Bibcode:1984Natur.308..667M. doi:10.1038/308667a0. S2CID 4372579.
- “Egg Allergy Facts” Archived 2013-01-12 at the Wayback Machine Asthma and Allergy Foundation of America
- Arnaldo Cantani (2008). Pediatric Allergy, Asthma and Immunology. Berlin: Springer. pp. 710–713. ISBN 978-3-540-20768-9.
- Roan, Shari (August 20, 2010). “Eggs and salmonella: What you need to know”. Los Angeles Times. Retrieved September 14, 2011.
- Dasgupta, Amitava (2019-01-01), Dasgupta, Amitava (ed.), “Chapter 2 – Biotin: Pharmacology, Pathophysiology, and Assessment of Biotin Status”, Biotin and Other Interferences in Immunoassays, Elsevier, pp. 17–35, ISBN 978-0-12-816429-7, retrieved 2020-08-27
- de Vandenesse, Urbain (2011). “Egg White”. Translated by Abigail Wendler Bainbridge. The Encyclopedia of Diderot & d’Alembert Collaborative Translation Project. Retrieved 31 March 2015.
Translation of “Blanc d’oeuf,” Encyclopédie ou Dictionnaire raisonné des sciences, des arts et des métiers, vol. 2. Paris, 1752
- Wine/Enology Notes #85, by Bruce Zoecklein, 22 Jan 2004, Virginia Cooperative Extension Service “Enology Notes #85 – Wine Enology Grape Chemistry Lab at Virginia Tech”. Archived from the original on 2006-09-01. Retrieved 2007-05-15.
- Fining & Clarifying Agents, by Terry Rayner Archived 2006-06-16 at the Wayback Machine
- “Quercetin – Quercetin – Anti-tumor Activity Helps Fight Cancer – Diet and Health.net”. diet-and-health.net. Archived from the original on 2007-09-28.
- “About Vegan Wine | What is it and why isn’t all wine vegan?”. Vegan Wine Box. Retrieved 2020-09-11.
- Smith, Stacey (16 January 2018). “10 best vegan wines”. The Independent. Retrieved 17 March 2021.
- Campbell, Heather R.; Alsharif, Fahd M.; Marsac, Patrick J.; Lodder, Robert A. (2020). “The Development of a Novel Pharmaceutical Formulation of D-Tagatose for Spray-Drying”. Journal of Pharmaceutical Innovation: 1–13. doi:10.1007/s12247-020-09507-4.
- A. S. Mujumdar (2007). Handbook of industrial drying. CRC Press. p. 710. ISBN 978-1-57444-668-5.
- “Contract Spray Dryer & Spray Drying Services | Elan”.
- Walter R. Niessen (2002). Combustion and incineration processes. CRC Press. p. 588. ISBN 978-0-8247-0629-6.
- Onwulata p.66
- Poozesh, Sadegh; Lu, Kun; Marsac, Patrick J. (July 2018). “On the particle formation in spray drying process for bio-pharmaceutical applications: Interrogating a new model via computational fluid dynamics”. International Journal of Heat and Mass Transfer. 122: 863–876. doi:10.1016/j.ijheatmasstransfer.2018.02.043.
- Ajay Kumar (2009). Bioseparation Engineering. I. K. International. p. 179. ISBN 978-93-8002-608-4.
- Onwulata pp.389–430
- Onwulata p.268
- Chiou, D.; Langrish, T. A. G. (2007). “Crystallization of Amorphous Components in Spray-Dried Powders”. Drying Technology. 25 (9): 1427–1435. doi:10.1080/07373930701536718.
- Heuzé V.; Tran G. (2016) [Last updated on March 31, 2016, 10:31]. “Blood meal”. Feedipedia. a programme by INRA, CIRAD, AFZ and FAO.
- Ting, Jeffrey M.; Porter, William W.; Mecca, Jodi M.; Bates, Frank S.; Reineke, Theresa M. (2018-01-10). “Advances in Polymer Design for Enhancing Oral Drug Solubility and Delivery”. Bioconjugate Chemistry. 29 (4): 939–952. doi:10.1021/acs.bioconjchem.7b00646. ISSN 1043-1802. PMID 29319295.
- Ricarte, Ralm G.; Van Zee, Nicholas J.; Li, Ziang; Johnson, Lindsay M.; Lodge, Timothy P.; Hillmyer, Marc A. (2019-09-05). “Recent Advances in Understanding the Micro- and Nanoscale Phenomena of Amorphous Solid Dispersions”. Molecular Pharmaceutics. 16 (10): 4089–4103. doi:10.1021/acs.molpharmaceut.9b00601. ISSN 1543-8384. PMID 31487183.
- Ornithology, Volume 1994 By Frank B. Gill p. 361
- James, John M.; Zeiger, Robert S.; Lester, Mitchell R.; Fasano, Mary Beth; Gern, James E.; Mansfield, Lyndon E.; Schwartz, Howard J.; Sampson, Hugh A.; Windom, Hugh H.; Machtinger, Steven B.; Lensing, Shelly (1998). “Safe administration of influenza vaccine to patients with egg allergy”. The Journal of Pediatrics. 133 (5): 624–8. doi:10.1016/S0022-3476(98)70101-5. PMID 9821418.
- McGee, Harold. On Food and Cooking: The Science and Lore of the Kitchen. New York: Scribner, 2004, edited by Vinay.[page needed]
- Exploratorium
- Takehiko Yamamoto, Mujo Kim (1996-12-13), Hen eggs, ISBN 9780849340055
- “Science of Cooking: Ask the Inquisitive Cooks!”. exploratorium.edu.
- McGee, Harold J.; Long, Sharon R.; Briggs, Winslow R. (1984). “Why whip egg whites in copper bowls?”. Nature. 308 (5960): 667–8. Bibcode:1984Natur.308..667M. doi:10.1038/308667a0. S2CID 4372579.
- “Egg Allergy Facts” Archived 2013-01-12 at the Wayback Machine Asthma and Allergy Foundation of America
- Arnaldo Cantani (2008). Pediatric Allergy, Asthma and Immunology. Berlin: Springer. pp. 710–713. ISBN 978-3-540-20768-9.
- Roan, Shari (August 20, 2010). “Eggs and salmonella: What you need to know”. Los Angeles Times. Retrieved September 14, 2011.
- Dasgupta, Amitava (2019-01-01), Dasgupta, Amitava (ed.), “Chapter 2 – Biotin: Pharmacology, Pathophysiology, and Assessment of Biotin Status”, Biotin and Other Interferences in Immunoassays, Elsevier, pp. 17–35, ISBN 978-0-12-816429-7, retrieved 2020-08-27
- de Vandenesse, Urbain (2011). “Egg White”. Translated by Abigail Wendler Bainbridge. The Encyclopedia of Diderot & d’Alembert Collaborative Translation Project. Retrieved 31 March 2015.
Translation of “Blanc d’oeuf,” Encyclopédie ou Dictionnaire raisonné des sciences, des arts et des métiers, vol. 2. Paris, 1752
- Gilbertus. Compendium Medicine Gilberti Anglici Tam Morborum Universalium Quam Particularium Nondum Medicis Sed & Cyrurgicis Utilissimum. Lugduni: Impressum per Jacobum Sacconum, expensis Vincentii de Portonariis, 1510.
- Good Eats, Let Them Eat Foam Archived 2021-01-27 at the Wayback Machine. DVD. Television Food Network, June 13, 2001.
- Marshall, F.A.S. Photography: the importance of its applications in preserving pictorial records. Containing a practical description of the Talbotype process (London: Hering & Remington; Peterborough, T Chadwell & J Clarke, 1855).
- MedlinePlus Encyclopedia: Food allergy
- Caubet JC, Wang J (2011). “Current understanding of egg allergy”. Pediatr. Clin. North Am. 58 (2): 427–43. doi:10.1016/j.pcl.2011.02.014. PMC 3069662. PMID 21453811.
- Soares-Weiser K, Takwoingi Y, Panesar SS, Muraro A, Werfel T, et al. (January 2014). “The diagnosis of food allergy: a systematic review and meta-analysis”. Allergy. 69 (1): 76–86. doi:10.1111/all.12333. PMID 24329961. S2CID 21493978.
- Ferraro V, Zanconato S, Carraro S (May 2019). “Timing of Food Introduction and the Risk of Food Allergy”. Nutrients. 11 (5): 1131. doi:10.3390/nu11051131. PMC 6567868. PMID 31117223.
- The EAACI Food Allergy and Anaphylaxis Guidelines Group (August 2014). “Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology”. Allergy. 69 (8): 1026–45. doi:10.1111/all.12437. PMID 24909803. S2CID 11054771.
- “Choosing Wisely: Don’t rely on antihistamines as firstline treatment in severe allergic reactions”. American Academy of Family Physicians. Retrieved 28 August 2022.
- Fineman, SM (July 2014). “Optimal Treatment of Anaphylaxis: Antihistamines Versus Epinephrine”. Postgraduate Medicine. 126 (4): 73–81. doi:10.3810/pgm.2014.07.2785. PMID 25141245. S2CID 25203272.
- Hasan SA, Wells RD, Davis CM (2013). “Egg hypersensitivity in review”. Allergy Asthma Proc. 34 (1): 26–32. doi:10.2500/aap.2013.34.3621. PMID 23406934.
- Urisu A, Kondo Y, Tsuge I (2015). “Hen’s Egg Allergy”. Food Allergy: Molecular Basis and Clinical Practice. Chemical Immunology and Allergy. Vol. 101. pp. 124–30. doi:10.1159/000375416. ISBN 978-3-318-02340-4. PMID 26022872.
- National Report of the Expert Panel on Food Allergy Research, NIH-NIAID 2003 “June 30 2003.pdf” (PDF). Archived from the original (PDF) on 2006-10-04. Retrieved 2006-08-07.
- “Food Allergy Facts” Archived 2012-10-06 at the Wayback Machine Asthma and Allergy Foundation of America
- Allen KJ, Turner PJ, Pawankar R, Taylor S, Sicherer S, Lack G, Rosario N, Ebisawa M, Wong G, Mills EN, Beyer K, Fiocchi A, Sampson HA (2014). “Precautionary labelling of foods for allergen content: are we ready for a global framework?”. World Allergy Organ J. 7 (1): 1–14. doi:10.1186/1939-4551-7-10. PMC 4005619. PMID 24791183.
- FDA (18 December 2017). “Food Allergies: What You Need to Know”. Food and Drug Administration. Retrieved 12 January 2018.
- “Agência Nacional de Vigilância Sanitária Guia sobre Programa de Controle de Alergênicos” (in Portuguese). Agência Nacional de Vigilância Sanitária (ANVISA). 2016. Archived from the original on 29 April 2018. Retrieved 7 April 2018.
- Ierodiakonou D, Garcia-Larsen V, Logan A, Groome A, Cunha S, Chivinge J, Robinson Z, Geoghegan N, Jarrold K, Reeves T, Tagiyeva-Milne N, Nurmatov U, Trivella M, Leonardi-Bee J, Boyle RJ (2016). “Timing of Allergenic Food Introduction to the Infant Diet and Risk of Allergic or Autoimmune Disease: A Systematic Review and Meta-analysis”. JAMA. 316 (11): 1181–1192. doi:10.1001/jama.2016.12623. hdl:10044/1/40479. PMID 27654604.
- Fiocchi A, Dahdah L, Bahna SL, Mazzina O, Assa’ad A (2016). “Doctor, when should I feed solid foods to my infant?”. Curr Opin Allergy Clin Immunol. 16 (4): 404–11. doi:10.1097/aci.0000000000000291. PMID 27327121. S2CID 36508449.
- Anderson J, Malley K, Snell R (2009). “Is 6 months still the best for exclusive breastfeeding and introduction of solids? A literature review with consideration to the risk of the development of allergies”. Breastfeed Rev. 17 (2): 23–31. PMID 19685855.
- Fiocchi A, Assa’ad A, Bahna S (2006). “Food allergy and the introduction of solid foods to infants: a consensus document. Adverse Reactions to Foods Committee, American College of Allergy, Asthma and Immunology”. Ann. Allergy Asthma Immunol. 97 (1): 10–20, quiz 21, 77. doi:10.1016/s1081-1206(10)61364-6. PMID 16892776.
- Urisu A, Ebisawa M, Ito K, Aihara Y, Ito S, Mayumi M, Kohno Y, Kondo N (2014). “Japanese Guideline for Food Allergy 2014”. Allergol Int. 63 (3): 399–419. doi:10.2332/allergolint.14-RAI-0770. PMID 25178179.
- “Egg Allergy Facts” Archived 2013-01-12 at the Wayback Machine Asthma and Allergy Foundation of America
- Sicherer SH, Sampson HA (2014). “Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment”. J Allergy Clin Immunol. 133 (2): 291–307. doi:10.1016/j.jaci.2013.11.020. PMID 24388012.
- Pols DH, Wartna JB, van Alphen EI, Moed H, Rasenberg N, Bindels PJ, Bohnen AM (2015). “Interrelationships between Atopic Disorders in Children: A Meta-Analysis Based on ISAAC Questionnaires”. PLOS ONE. 10 (7): e0131869. Bibcode:2015PLoSO..1031869P. doi:10.1371/journal.pone.0131869. PMC 4489894. PMID 26135565.
- “Recommendations for the production and control of influenza vaccine (inactivated)” (PDF). World Health Organization. Archived (PDF) from the original on October 28, 2013. Retrieved May 27, 2013.
- Grohskopf LA, Sokolow LZ, Broder KR; et al. (2017). “Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2017–18 Influenza Season”. MMWR Recomm Rep. 66 (2): 1–20. doi:10.15585/mmwr.rr6602a1. PMC 5837399. PMID 28841201.
- Committee on Infectious Diseases, American Academy of Pediatrics (2016). “Recommendations for Prevention and Control of Influenza in Children, 2016-2017”. Pediatrics. 138 (4): e20162527. doi:10.1542/peds.2016-2527. PMID 27600320.
- Committee On Infectious Diseases, American Academy of Pediatrics (2015). “Recommendations for Prevention and Control of Influenza in Children, 2015-2016”. Pediatrics. 136 (4): 792–808. doi:10.1542/peds.2015-2920. PMID 26347430.
- Committee on Infectious Diseases, American Academy of Pediatrics (2011). “Recommendations for prevention and control of influenza in children, 2011-2012”. Pediatrics. 128 (4): 813–25. doi:10.1542/peds.2011-2295. PMID 21890834.
- Piquer-Gibert M, Plaza-Martín A, Martorell-Aragonés A, Ferré-Ybarz L, Echeverría-Zudaire L, Boné-Calvo J, Nevot-Falcó S (2007). “Recommendations for administering the triple viral vaccine and anti-influenza vaccine in patients with egg allergy”. Allergol Immunopathol (Madr). 35 (5): 209–12. doi:10.1157/13110316. PMID 17923075. S2CID 10902757.
- Clark AT, Skypala I, Leech SC, Ewan PW, Dugué P, Brathwaite N, Huber PA, Nasser SM (2010). “British Society for Allergy and Clinical Immunology guidelines for the management of egg allergy”. Clin. Exp. Allergy. 40 (8): 1116–29. doi:10.1111/j.1365-2222.2010.03557.x. PMID 20649608. S2CID 29950268.
- Feldweg AM (2017). “Food-Dependent, Exercise-Induced Anaphylaxis: Diagnosis and Management in the Outpatient Setting”. J Allergy Clin Immunol Pract. 5 (2): 283–288. doi:10.1016/j.jaip.2016.11.022. PMID 28283153.
- Pravettoni V, Incorvaia C (2016). “Diagnosis of exercise-induced anaphylaxis: current insights”. J Asthma Allergy. 9: 191–198. doi:10.2147/JAA.S109105. PMC 5089823. PMID 27822074.
- Kim CW, Figueroa A, Park CH, Kwak YS, Kim KB, Seo DY, Lee HR (2013). “Combined effects of food and exercise on anaphylaxis”. Nutr Res Pract. 7 (5): 347–51. doi:10.4162/nrp.2013.7.5.347. PMC 3796658. PMID 24133612.
- “Food allergy”. NHS Choices. 16 May 2016. Retrieved 31 January 2017.
A food allergy is when the body’s immune system reacts unusually to specific foods
- Food Reactions. Allergies Archived 2010-04-16 at the Wayback Machine. Foodreactions.org. Kent, England. 2005. Accessed 27 Apr 2010.
- Davis PJ, Williams SC (1998). “Protein modification by thermal processing”. Allergy. 53 (46 Suppl): 102–5. doi:10.1111/j.1398-9995.1998.tb04975.x. PMID 9826012. S2CID 10621652.
- Verhoeckx KC, Vissers YM, Baumert JL, Faludi R, Feys M, Flanagan S, Herouet-Guicheney C, Holzhauser T, Shimojo R, van der Bolt N, Wichers H, Kimber I (June 2015). “Food processing and allergenicity”. Food Chem Toxicol. 80: 223–240. doi:10.1016/j.fct.2015.03.005. PMID 25778347.
- Janeway, Charles; Paul Travers; Mark Walport; Mark Shlomchik (2001). Immunobiology; Fifth Edition. New York and London: Garland Science. pp. e–book. ISBN 978-0-8153-4101-7. Archived from the original on 2009-06-28.
- Grimbaldeston MA, Metz M, Yu M, Tsai M, Galli SJ (2006). “Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune responses”. Curr. Opin. Immunol. 18 (6): 751–60. doi:10.1016/j.coi.2006.09.011. PMID 17011762.
- Holt PG, Sly PD (2007). “Th2 cytokines in the asthma late-phase response”. Lancet. 370 (9596): 1396–8. doi:10.1016/S0140-6736(07)61587-6. PMID 17950849. S2CID 40819814.
- Arnaldo Cantani (2008). Pediatric Allergy, Asthma and Immunology. Berlin: Springer. pp. 710–713. ISBN 978-3-540-20768-9.
- Joris, Isabelle; Majno, Guido (2004). Cells, tissues, and disease: principles of general pathology. Oxford [Oxfordshire]: Oxford University Press. p. 538. ISBN 978-0-19-514090-3.
- Carina Venter; Isabel Skypala (2009). Food Hypersensitivity: Diagnosing and Managing Food Allergies and Intolerance. Wiley-Blackwell. pp. 129–131. ISBN 978-1-4051-7036-9.
- Soares-Weiser K, Takwoingi Y, Panesar SS, Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Halken S, Poulsen L, van Ree R, Vlieg-Boerstra BJ, Sheikh A (2014). “The diagnosis of food allergy: a systematic review and meta-analysis”. Allergy. 69 (1): 76–86. doi:10.1111/all.12333. PMID 24329961.
- Calvani M, Arasi S, Bianchi A, Caimmi D, Cuomo B, Dondi A, Indirli GC, La Grutta S, Panetta V, Verga MC (2015). “Is it possible to make a diagnosis of raw, heated, and baked egg allergy in children using cutoffs? A systematic review”. Pediatr Allergy Immunol. 26 (6): 509–21. doi:10.1111/pai.12432. hdl:10447/143352. PMID 26102461. S2CID 206241392.
- Martorell A, Alonso E, Boné J, Echeverría L, López MC, Martín F, et al. (2013). “Position document: IgE-mediated allergy to egg protein”. Allergol Immunopathol (Madr) (Review). 41 (5): 320–36. doi:10.1016/j.aller.2013.03.005. PMID 23830306.
- Tang AW (2003). “A practical guide to anaphylaxis”. Am Fam Physician. 68 (7): 1325–1332. PMID 14567487.
- Romantsik, O; Tosca, MA; Zappettini, S; Calevo, MG (20 April 2018). “Oral and sublingual immunotherapy for egg allergy”. The Cochrane Database of Systematic Reviews. 2018 (4): CD010638. doi:10.1002/14651858.CD010638.pub3. PMC 6494514. PMID 29676439.
- Ibáñez MD, Escudero C, Sánchez-García S, Rodríguez del Río P (2015). “Comprehensive Review of Current Knowledge on Egg Oral Immunotherapy”. J Investig Allergol Clin Immunol. 25 (5): 316–28, quiz 2 p following 328. PMID 26727760.
- Lambert R, Grimshaw KE, Ellis B, Jaitly J, Roberts G (2017). “Evidence that eating baked egg or milk influences egg or milk allergy resolution: a systematic review”. Clin. Exp. Allergy. 47 (6): 829–837. doi:10.1111/cea.12940. PMID 28516451. S2CID 5207549.
- Carey, John (1997). Eyewitness to Science. Harvard University Press. p. 173. ISBN 9780674287556.
- Nanagas, VC; Baldwin, JL; Karamched, KR (July 2017). “Hidden Causes of Anaphylaxis”. Current Allergy and Asthma Reports. 17 (7): 44. doi:10.1007/s11882-017-0713-2. PMID 28577270. S2CID 33691910.
- Peters RL, Dharmage SC, Gurrin LC, Koplin JJ, Ponsonby AL, Lowe AJ, Tang ML, Tey D, Robinson M, Hill D, Czech H, Thiele L, Osborne NJ, Allen KJ (2014). “The natural history and clinical predictors of egg allergy in the first 2 years of life: a prospective, population-based cohort study”. J. Allergy Clin. Immunol. 133 (2): 485–91. doi:10.1016/j.jaci.2013.11.032. PMID 24373356. S2CID 24028314.
- Arik Yilmaz E, Cavkaytar O, Buyuktiryaki B, Sekerel BE, Soyer O, Sackesen C (2015). “Factors associated with the course of egg allergy in children”. Ann. Allergy Asthma Immunol. 115 (5): 434–438.e1. doi:10.1016/j.anai.2015.08.012. PMID 26505933.
- Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A (2014). “Prevalence of common food allergies in Europe: a systematic review and meta-analysis”. Allergy. 69 (8): 992–1007. doi:10.1111/all.12423. PMID 24816523. S2CID 28692645.
- Gray CL (2017). “Food Allergy in South Africa”. Curr Allergy Asthma Rep. 17 (6): 35. doi:10.1007/s11882-017-0703-4. PMID 28470372. S2CID 44840606.
- “What is Prevalence?” National Institute of Mental Health (Accessed 25 December 2020).
- Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, Massing M, Cohn RD, Zeldin DC (2010). “National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006”. J. Allergy Clin. Immunol. 126 (4): 798–806.e13. doi:10.1016/j.jaci.2010.07.026. PMC 2990684. PMID 20920770.
- Unsel M, Sin AZ, Ardeniz O, Erdem N, Ersoy R, Gulbahar O, Mete N, Kokuludağ A (2007). “New onset egg allergy in an adult”. J Investig Allergol Clin Immunol. 17 (1): 55–8. PMID 17323866.
- Ravid NL, Annunziato RA, Ambrose MA, Chuang K, Mullarkey C, Sicherer SH, Shemesh E, Cox AL (2015). “Mental health and quality-of-life concerns related to the burden of food allergy”. Psychiatr. Clin. North Am. 38 (1): 77–89. doi:10.1016/j.psc.2014.11.004. PMID 25725570.
- Morou Z, Tatsioni A, Dimoliatis ID, Papadopoulos NG (2014). “Health-related quality of life in children with food allergy and their parents: a systematic review of the literature”. J Investig Allergol Clin Immunol. 24 (6): 382–95. PMID 25668890.
- Lange L (2014). “Quality of life in the setting of anaphylaxis and food allergy”. Allergo J Int. 23 (7): 252–260. doi:10.1007/s40629-014-0029-x. PMC 4479473. PMID 26120535.
- van der Velde JL, Dubois AE, Flokstra-de Blok BM (2013). “Food allergy and quality of life: what have we learned?”. Curr Allergy Asthma Rep. 13 (6): 651–61. doi:10.1007/s11882-013-0391-7. PMID 24122150. S2CID 326837.
- Culinary Institute of America Allergen-free oasis comes to the CIA (2017)
- Shah E, Pongracic J (2008). “Food-induced anaphylaxis: who, what, why, and where?”. Pediatr Ann. 37 (8): 536–41. doi:10.3928/00904481-20080801-06. PMID 18751571.
- “Food Allergen Labeling and Consumer Protection Act of 2004”. FDA. 2 August 2004. Retrieved 7 March 2022.
- “Food allergen labelling and information requirements under the EU Food Information for Consumers Regulation No. 1169/2011: Technical Guidance” (April 2015).
- FDA (14 December 2017). “Have Food Allergies? Read the Label”. Food and Drug Administration. Retrieved 14 January 20
- “Food Ingredients of Public Health Concern” (PDF). United States Department of Agriculture. Food Safety and Inspection Service. 7 March 2017. Retrieved 16 February 2018.
- “Allergies and Food Safety”. United States Department of Agriculture. Food Safety and Inspection Service. 1 December 2016. Retrieved 16 February 2018.
- Roses JB (2011). “Food allergen law and the Food Allergen Labeling and Consumer Protection Act of 2004: falling short of true protection for food allergy sufferers”. Food Drug Law J. 66 (2): 225–42, ii. PMID 24505841.
- FDA (18 July 2006). “Food Allergen Labeling And Consumer Protection Act of 2004 Questions and Answers”. Food and Drug Administration. Retrieved 12 March 2018.
- “Allergy and intolerance: guidance for businesses”. Archived from the original on 2014-12-08. Retrieved 2014-12-12.
- Mills EN, Valovirta E, Madsen C, Taylor SL, Vieths S, Anklam E, Baumgartner S, Koch P, Crevel RW, Frewer L (2004). “Information provision for allergic consumers–where are we going with food allergen labelling?”. Allergy. 59 (12): 1262–1268. doi:10.1111/j.1398-9995.2004.00720.x. PMID 15507093. S2CID 40395908.
- Taylor SL, Baumert JL (2015). “Worldwide food allergy labeling and detection of allergens in processed foods”. Food Allergy: Molecular Basis and Clinical Practice. Chemical Immunology and Allergy. Vol. 101. pp. 227–234. doi:10.1159/000373910. ISBN 978-3-318-02340-4. PMID 26022883.
{{cite book}}:|journal=ignored (help) - DunnGalvin A, Chan CH, et al. (2015). “Precautionary allergen labelling: perspectives from key stakeholder groups”. Allergy. 70 (9): 1039–1051. doi:10.1111/all.12614. PMID 25808296. S2CID 18362869.
- Zurzolo GA, de Courten M, Koplin J, Mathai ML, Allen KJ (2016). “Is advising food allergic patients to avoid food with precautionary allergen labelling out of date?”. Curr Opin Allergy Clin Immunol. 16 (3): 272–277. doi:10.1097/ACI.0000000000000262. PMID 26981748. S2CID 21326926.
- Allen KJ, Remington BC, Baumert JL, Crevel RW, Houben GF, Brooke-Taylor S, Kruizinga AG, Taylor SL (2014). “Allergen reference doses for precautionary labeling (VITAL 2.0): clinical implications”. J. Allergy Clin. Immunol. 133 (1): 156–164. doi:10.1016/j.jaci.2013.06.042. PMID 23987796.
- Taylor SL, Baumert JL, Kruizinga AG, Remington BC, Crevel RW, Brooke-Taylor S, Allen KJ, Houben G (2014). “Establishment of Reference Doses for residues of allergenic foods: report of the VITAL Expert Panel”. Food Chem. Toxicol. 63: 9–17. doi:10.1016/j.fct.2013.10.032. PMID 24184597.
- The VITAL Program Allergen Bureau, Australia and New Zealand.
- Popping B, Diaz-Amigo C (2018). “European Regulations for Labeling Requirements for Food Allergens and Substances Causing Intolerances: History and Future”. J AOAC Int. 101 (1): 2–7. doi:10.5740/jaoacint.17-0381. PMID 29202901.
- Fong AT, Katelaris CH, Wainstein B (2017). “Bullying and quality of life in children and adolescents with food allergy”. J Paediatr Child Health. 53 (7): 630–635. doi:10.1111/jpc.13570. PMID 28608485. S2CID 9719096
- “Povidones, Copovidones, and Crospovidones for Pharmaceutical Products”. BASF Pharma. Retrieved 2022-06-11.
- Microsoft Word – G0294.doc[dead link]
- “A Novel Stabilization of Beer with Polyclar Brewbrite. Mustafa Rehmanji, Chandra Gopal, and Andrew Mola, MBAA TQ vol. 39, no. 1, 2002, pp. 24–28” (PDF). Archived from the original (PDF) on 2015-09-06. Retrieved 2017-05-19.
- Hammerstone JF, Lazarus SA, Schmitz HH (August 2000). “Procyanidin content and variation in some commonly consumed foods”. The Journal of Nutrition. 130 (8S Suppl): 2086S–2092S. doi:10.1093/jn/130.8.2086S. PMID 10917927.
- Payne MJ, Hurst WJ, Miller KB, Rank C, Stuart DA (October 2010). “Impact of fermentation, drying, roasting, and Dutch processing on epicatechin and catechin content of cacao beans and cocoa ingredients”. Journal of Agricultural and Food Chemistry. 58 (19): 10518–10527. doi:10.1021/jf102391q. PMID 20843086.
- Mabrym H, Harborne JB, Mabry TJ (1975). The Flavonoids. London: Chapman and Hall. ISBN 978-0-412-11960-6.
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (May 2004). “Polyphenols: food sources and bioavailability”. The American Journal of Clinical Nutrition. 79 (5): 727–747. doi:10.1093/ajcn/79.5.727. PMID 15113710.
- Del Río D, Rodríguez Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A (May 2013). “Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases”. Antioxidants & Redox Signaling. 18 (14): 1818–1892. doi:10.1089/ars.2012.4581. PMC 3619154. PMID 22794138.
- Rodríguez Mateos A, Weber T, Skene SS, Ottaviani JI, Crozier A, Kelm M, et al. (December 2018). “Assessing the respective contributions of dietary flavanol monomers and procyanidins in mediating cardiovascular effects in humans: randomized, controlled, double-masked intervention trial”. The American Journal of Clinical Nutrition. 108 (6): 1229–1237. doi:10.1093/ajcn/nqy229. PMC 6290365. PMID 30358831.
- Actis-Goretta L, Lévèques A, Rein M, Teml A, Schäfer C, Hofmann U, et al. (October 2013). “Intestinal absorption, metabolism, and excretion of (−)-epicatechin in healthy humans assessed by using an intestinal perfusion technique”. The American Journal of Clinical Nutrition. 98 (4): 924–933. doi:10.3945/ajcn.113.065789. PMID 23864538.
- Kuhnle G, Spencer JP, Schroeter H, Shenoy B, Debnam ES, Srai SK, et al. (October 2000). “Epicatechin and catechin are O-methylated and glucuronidated in the small intestine”. Biochemical and Biophysical Research Communications. 277 (2): 507–512. doi:10.1006/bbrc.2000.3701. PMID 11032751.
- Das NP (December 1971). “Studies on flavonoid metabolism. Absorption and metabolism of (+)-catechin in man”. Biochemical Pharmacology. 20 (12): 3435–3445. doi:10.1016/0006-2952(71)90449-7. PMID 5132890.
- Ottaviani JI, Borges G, Momma TY, et al. (July 2016). “The metabolome of [2-14C](−)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives”. Scientific Reports. 6 (1): 29034. Bibcode:2016NatSR…629034O. doi:10.1038/srep29034. PMC 4929566. PMID 27363516.
- Ottaviani JI, Fong R, Kimball J, Ensunsa JL, Britten A, Lucarelli D, et al. (June 2018). “Evaluation at scale of microbiome-derived metabolites as biomarker of flavan-3-ol intake in epidemiological studies”. Scientific Reports. 8 (1): 9859. Bibcode:2018NatSR…8.9859O. doi:10.1038/s41598-018-28333-w. PMC 6026136. PMID 29959422.
- Health Canada (12 December 2017). “Summary Safety Review – Green tea extract-containing natural health products – Assessing the potential risk of liver injury (hepatotoxicity)”. Health Canada, Government of Canada. Retrieved 2022-05-06.
- Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, et al. (April 2018). “Scientific opinion on the safety of green tea catechins”. EFSA Journal. 16 (4): e05239. doi:10.2903/j.efsa.2018.5239. PMC 7009618. PMID 32625874.
- Ullah C, Unsicker SB, Fellenberg C, Constabel CP, Schmidt A, Gershenzon J, Hammerbacher A (December 2017). “Flavan-3-ols Are an Effective Chemical Defense against Rust Infection”. Plant Physiology. 175 (4): 1560–1578. doi:10.1104/pp.17.00842. PMC 5717727. PMID 29070515.
- Schwitters B, Masquelier J (1995). OPC in Practice (3rd ed.). OCLC 45289285.
- Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A (May 2013). “Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases”. Antioxidants & Redox Signaling. 18 (14): 1818–1892. doi:10.1089/ars.2012.4581. PMC 3619154. PMID 22794138.
- Dewick PM (2009). Medicinal Natural Products: a biosynthetic approach. John Wiley & Sons. p. 168. ISBN 978-0-471-49641-0.
- Winkel-Shirley B (June 2001). “Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology”. Plant Physiology. 126 (2): 485–493. doi:10.1104/pp.126.2.485. PMC 1540115. PMID 11402179.
- Mueller-Harvey I, Feucht W, Polster J, Trnková L, Burgos P, Parker AW, Botchway SW (March 2012). “Two-photon excitation with pico-second fluorescence lifetime imaging to detect nuclear association of flavanols”. Analytica Chimica Acta. 719: 68–75. doi:10.1016/j.aca.2011.12.068. PMID 22340533. S2CID 24094780.
- “Database on polyphenol content in foods, v. 3.6”. Phenol Explorer. 2016.
- Ottaviani JI, Borges G, Momma TY, Spencer JP, Keen CL, Crozier A, Schroeter H (July 2016). “The metabolome of [2-14C](−)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives”. Scientific Reports. 6: 29034. Bibcode:2016NatSR…629034O. doi:10.1038/srep29034. PMC 4929566. PMID 27363516.
- Actis-Goretta L, Lévèques A, Rein M, Teml A, Schäfer C, Hofmann U, et al. (October 2013). “Intestinal absorption, metabolism, and excretion of (−)-epicatechin in healthy humans assessed by using an intestinal perfusion technique”. The American Journal of Clinical Nutrition. 98 (4): 924–933. doi:10.3945/ajcn.113.065789. PMID 23864538.
- Ottaviani JI, Momma TY, Kuhnle GK, Keen CL, Schroeter H (April 2012). “Structurally related (−)-epicatechin metabolites in humans: assessment using de novo chemically synthesized authentic standards”. Free Radical Biology & Medicine. 52 (8): 1403–1412. doi:10.1016/j.freeradbiomed.2011.12.010. PMID 22240152.
- “Flavonoids”. Linus Pauling Institute, Oregon State University, Corvallis. 2016. Retrieved 24 July 2016.
- Ottaviani JI, Momma TY, Heiss C, Kwik-Uribe C, Schroeter H, Keen CL (January 2011). “The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo”. Free Radical Biology & Medicine. 50 (2): 237–244. doi:10.1016/j.freeradbiomed.2010.11.005. PMID 21074608.
- Cermak R, Wolffram S (October 2006). “The potential of flavonoids to influence drug metabolism and pharmacokinetics by local gastrointestinal mechanisms”. Curr. Drug Metab. 7 (7): 729–44. doi:10.2174/138920006778520570. PMID 17073577. Archived from the original on 2012-07-20.
- Si D, Wang Y, Zhou YH, et al. (March 2009). “Mechanism of CYP2C9 inhibition by flavones and flavonols”. Drug Metab. Dispos. 37 (3): 629–34. doi:10.1124/dmd.108.023416. PMID 19074529. S2CID 285706.
- Holland, Thomas Monroe; Agarwal, Puja; Wang, Yamin; Dhana, Klodian; Leurgans, Sue E.; Shea, Kyla; Booth, Sarah L; Rajan, Kumar; Schneider, Julie A.; Barnes, Lisa L. (22 November 2022). “Association of Dietary Intake of Flavonols With Changes in Global Cognition and Several Cognitive Abilities”. Neurology. 100 (7): e694–e702. doi:10.1212/WNL.0000000000201541. PMC 9969915. S2CID 253800625.
Further reading
- Cook, E.M, and DuMont, H.D. (1991) Process Drying Practice, McGraw-Hill, Inc., ISBN 0-07-012462-0
- Keey, R.B., (1992). Drying of Loose and Particulate Materials 1st ed., Taylor & Francis, ISBN 0-89116-878-8
- Nutritional evaluation of food processing second edition (1975), Robert S. Harris, Ph.D. and Endel Karmas Ph.D. (eds)
- Filková, I., & Mujumdar, A. S. (2020). Industrial spray drying systems. In Handbook of industrial drying (pp. 263-307). CRC Press.
- Jafari, S. M., Arpagaus, C., Cerqueira, M. A., & Samborska, K. (2021). Nano spray drying of food ingredients; materials, processing and applications. Trends in Food Science & Technology, 109, 632-646.
- Klimša, V., Ruphuy, G., Jonáš, J., Mašková, L., Kašpar, O., Žvátora, P., & Štěpánek, F. (2023). Spray drying robot for high-throughput combinatorial fabrication of multicomponent solid dispersions. Powder Technology, 428, 118872.
External links
- George Eastman House “Photographic Process 3.0: The Albumen Process” (archived 27 October 2016)
- Old Photos of Japan — Samples of hand-tinted albumen photographs Archived 27 April 2018 at the Wayback Machine
- Albumen prints from the American University in Cairo Rare Books and Special Collections Digital Library (archived 7 November 2012)
- Albumen Photographs: history, science and preservation
- Jarvis, Chad. “Albumen printing: Creating and processing albumen paper”. Alternativephotography.com. Archived from the original on 14 July 2012. Retrieved 8 July 2012.
- “Kiwi Sun Photography: Albumen Printing”. Archived from the original on 7 January 2009.
- Photos of Japan — A collection of hand-painted Japanese albumen prints
- Albumen prints from the University of Michigan Museum of Art
- Enology Notes #46, by Bruce Zoecklein, Virginia Cooperative Extension Service, 17 May 2002
- Bentonite Fining of Juice and Wine, by Bruce Zoecklein, Virginia Cooperative Extension Service, pub. 463-014, 1988
- Common Wine and Beer Finings
- Colloidal stabilisation of beer, The Brewer International, Jan 2002
- Fining Agents for Wine Archived 2006-09-13 at the Wayback Machine, by J.R. Morris and G.L. Main, Proceedings of the 14th NM Conference, 1995]
- Fining, by Ben Rotter
- The Use of Gelatin In Wine Fining, by C.G.B. Cole
- Animation of standard Spray Drying Concept
- Spray Drying trainings paper
| Photography |
|---|
Wikibooks Cookbook has a recipe/module on
Look up albumen or glair in Wiktionary, the free dictionary.







Leave a Reply