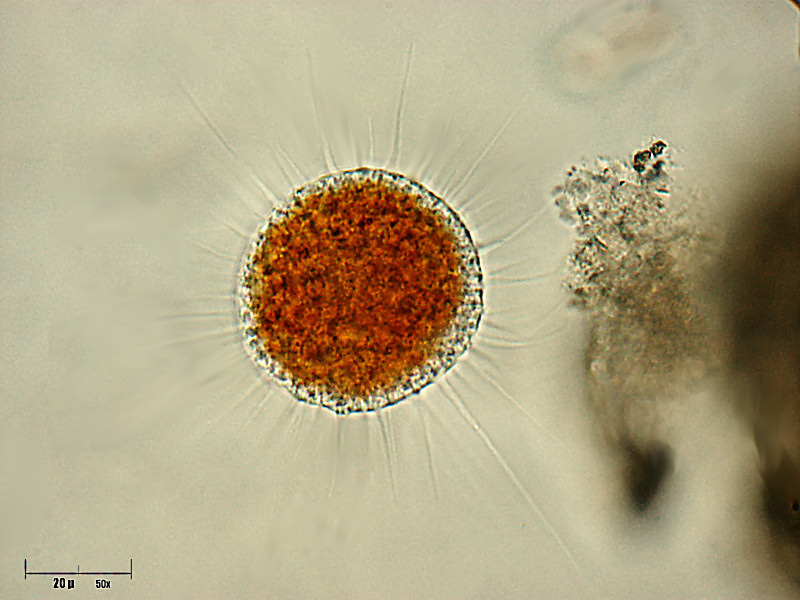
Vampyrella mitosis
Orthomitosis in Vampyrella occurs late in the cyst stage. Neither microtubule organizing centers (MTOCs) nor centrioles are present during mitosis. While in the trophozoite life stage and early cyst stage, the cell is in interphase. Heterochromatin decrease upon entering the cyst stage as the cell prepares for mitosis. The spherical nuclei increase in size from 1.5-2.0 µm in the trophozoite stage to 2.5-3.5 µm. The pars granulosa becomes more prominent within the cyst and the components begin to mix with the pars fibrosa. During prophase, chromosomes are condensed and bundles of microtubules appear on either side of the nuclei. The nucleolus begins to disintegrate within the nucleus. In metaphase, the nucleus takes on a cylindrical shape. Centric mitotic spindles do not reach the poles of the nucleus. The compacted chromosomes then create an equatorial plate with microtubules running through the plate. No kinetochores are present. Vesicles begin to accumulate near the chromosomes and the inner membrane of the intact nuclear envelope. The chromatids separate and the nuclei elongate in anaphase. This is followed by an increase in vesicles on the inner membrane. During telophase, the nuclei form a dumbbell shape. Vesicles begin to fuse around the chromosomes to form a new nuclear envelope as the older envelope disintegrates. After karyokinesis, the cell undergoes cytokinesis. At this point the nuclei are already spherical and resemble that of mature trophozoites.
- RÖPSTORF, P., HÜLSMANN, N., & HAUSMANN, K. (1994). Comparative fine structural investigations of interphase and mitotic nuclei of vampyrellid filose amoebae. The Journal of Eukaryotic Microbiology, 41(1), 18-30. doi:10.1111/j.1550-7408.1994.tb05930.x
For the comic book character, see Vampirella.

Vampyrella is a genus of amoebae belonging to the vampyrellid cercozoans usually ranging from 30-60 µm. Members of the genus alternate between two life stages: a free-living trophozoite stage and a cyst stage in which mitosis occurs. This taxon has received a great deal of attention due to their peculiar feeding behaviour of perforating the cell wall of algal cells and drawing out the contents for nourishment.
- RÖPSTORF, P., HÜLSMANN, N., & HAUSMANN, K. (1994). Comparative fine structural investigations of interphase and mitotic nuclei of vampyrellid filose amoebae. The Journal of Eukaryotic Microbiology, 41(1), 18-30. doi:10.1111/j.1550-7408.1994.tb05930.x
History
Vampire amoebae were first discovered in 1865 by Leon Cienkowski. These amoebae were given the genus name Vampyrella due to their bright red colouration and their distinct feeding habits in which they perforate the cell wall of their host and draw out the inner contents of the cell, resembling that of folklore vampires.
- Cienkowski, L. (1865). Beiträge zur kenntniss der monaden. Archiv Für Mikroskopische Anatomie, 1(1), 203–232. doi:10.1007/BF02961414
At present, the vampyrellids are placed taxonomically in the cercozoan subphylum Endomyxa along with some soil dwelling taxa. The clade within Endomyxa, Vampyrellida West, 1901, consists of the genera Theratomyxa, Platyreta, ‘Arachnula’, Leptophrys and Vampyrella. Molecular sequence data exist for two families: Vampyrellidae and Leptophyridae. Frequently, Vampyrella is the only genus represented in Vampyrellidae.
- Hess, S., Sausen, N., & Melkonian, M. (2012). Shedding light on vampires: The phylogeny of vampyrellid amoebae revisited. PLOS One, 7(2), e31165. doi:10.1371/journal.pone.0031165
Organism and life stages
Most members of Vampyrella obligatorily alternate between a free living trophozoite stage and cyst stage. In the trophozoite stage amoebae are free moving. Vampyrella is characterized by a roughly spherical shape, however, it often changes shape and becomes more ellipsoid or oblong. A narrow, colourless ectoplasm at the edge of the cells can be very easily differentiated from the intensely coloured cell body. The central body tends to vary between brick red, orange, reddish yellow, brown, or green. Numerous long colourless pseudopodia extend in all directions giving trophozoites an isodiametric morphotype. These pseudopodia can be as long as three times the diameter of the cell body and may be branched or unbranched. The pseudopodia may accumulate in the direction in which the organism moves. Shorter, slow moving, pin-like pseudopodia are projected and withdrawn very quickly from the cell body in addition to having longer pseudopodia. Many vacuoles can sometimes be seen in the periphery of the organi in addition to large, bubble-like non-contractile vacuoles. Young trophozoites range from about 25-40µm, while mature trophozoites can reach to about 70µm. Organisms tend to have multiple spherical nuclei with a central compact nucleolus. Some species of Vampyrella have been observed to fuse with each other to form large plasmodia with the same structure and colouration as a single free-living cell. This has been observed when food has been limited.
- RÖPSTORF, P., HÜLSMANN, N., & HAUSMANN, K. (1994). Comparative fine structural investigations of interphase and mitotic nuclei of vampyrellid filose amoebae. The Journal of Eukaryotic Microbiology, 41(1), 18-30. doi:10.1111/j.1550-7408.1994.tb05930.x
- Hess, S., Sausen, N., & Melkonian, M. (2012). Shedding light on vampires: The phylogeny of vampyrellid amoebae revisited. PLOS One, 7(2), e31165. doi:10.1371/journal.pone.0031165
- Leidy, J., Biodiversity Heritage Library, & Canadian Libraries. (1879). Fresh-water rhizopods of north America. p. 253-256.
- West, G. S. (1901). On some british freshwater rhizopods and heliozoa. Journal of the Linnean Society of London, Zoology, 28(183), 308-342. doi:10.1111/j.1096-3642.1901.tb01754.x
- Hoogenraad HR (1927) Bemerkungen über das Genus Leptophrys Hertw. et Lesser. Biol Zentralbl 47: 517–536.
- impano, P., & Pfiester, L. A. (1986). Observations on “Vampyrella penula-Stylodinium sphaera” and the ultrastructure of the reproductive cyst. American Journal of Botany, 73(9), 1341-1350. doi:10.2307/2444068
A plasmodium is a living structure of cytoplasm that contains many nuclei, rather than being divided into individual cells each with a single nucleus. Plasmodia are best known from slime molds, but are also found in parasitic Myxosporea, and some algae such as the Chlorarachniophyta. A plasmodium is an amoeboid, multinucleate, and naked mass of cytoplasm that contains many diploid nuclei. The resulting structure, a coenocyte, is created by many nuclear divisions without the process of cytokinesis, which in other organisms pulls newly-divided cells apart. In some cases, the resulting structure is a syncytium, created by the fusion of cells after division. Under suitable conditions, plasmodia differentiates and forms fruiting bodies bearing spores at their tips. The term plasmodium, introduced by Leon Cienkowski, usually refers to the feeding stage of slime molds; these are macroscopic mycetozoans. The multinucleate developmental stages of some intracellular parasites, namely Microsporidia (now in Fungi) and Myxosporidia (now in Cnidaria), former cnidosporans, are also sometimes called plasmodia. Similarly, in Rhizaria, the amoeboid, multinucleate protoplasts of some Cercozoan algae, e.g. Chlorarachniophyta, are called plasmodia. These lack cell walls; the syncytia are created by cell fusion. Some plasmodiophorids and haplosporidians are other multinucleated rhizarians.
- Sharma OP. (1988). “4. Myxomycota”. Textbook of Fungi. Boston: McGraw Hill Higher Education. pp. 33–48. ISBN 978-0-07-460329-1. Retrieved January 31, 2013.
- Kuznicki, L. & Dryl, S. (1987). Leon Cienkowski. Acta Protozoologica 26 (1): 1-2, [1].
- Berg, Linda (2008). Introductory Botany: Plants, People, and the Environment (2nd ed.). Belmont CA: Thomson Corporation. p. 398. ISBN 978-0-03-075453-1.
- Hoek, C. van den, Mann, D.G. and Jahns, H.M. (1995). Algae An Introduction to Phycology. Cambridge University Press, Cambridge. ISBN 0-521-30419-9.
- Brown MW, Kolisko M, Silberman JD, Roger AJ. (2012). Aggregative Multicellularity Evolved Independently in the Eukaryotic Supergroup Rhizaria. Current Biology, Volume 22, Issue 12, 1123-1127.
For the genus of parasitic protozoa which causes malaria, see Plasmodium.
Cyst stage
Following the trophozoite stage, cells enter an obligatory digestive and a later reproductive cyst stage. Cysts tend to appear roundish or elliptical and flattened on the side attached to a substrate. Cysts range from 50 to 100um in size. During digestion the cyst will turn from green to red, orange or brown colours. The cyst is covered in two envelopes. The outer envelope is softer and used to attach to a substrate such as filamentous food (algae). A stalk may or may not be present. The inner envelope is stronger and surrounds the cell while cell division takes place. After cell division, the daughter cells leave the cyst envelope leaving behind food remnants. During unfavourable conditions the cell may enter into a resting phase. Sex is currently unknown in the genus.
- RÖPSTORF, P., HÜLSMANN, N., & HAUSMANN, K. (1994). Comparative fine structural investigations of interphase and mitotic nuclei of vampyrellid filose amoebae. The Journal of Eukaryotic Microbiology, 41(1), 18-30. doi:10.1111/j.1550-7408.1994.tb05930.x
- Hess, S., Sausen, N., & Melkonian, M. (2012). Shedding light on vampires: The phylogeny of vampyrellid amoebae revisited. PLOS One, 7(2), e31165. doi:10.1371/journal.pone.0031165
Habitat and predation
Vampyrellids can be found in a wide variety of habitats, soil, freshwater or marine, but members of Vampyrella tend to be found in freshwater environments.
Vampire amoebae of this genus are heterotrophic and feed on algae, notably Spirogyra but also Chaetophora or Mougeotia.
- RÖPSTORF, P., HÜLSMANN, N., & HAUSMANN, K. (1994). Comparative fine structural investigations of interphase and mitotic nuclei of vampyrellid filose amoebae. The Journal of Eukaryotic Microbiology, 41(1), 18-30. doi:10.1111/j.1550-7408.1994.tb05930.x
- Hoogenraad HR (1927) Bemerkungen über das Genus Leptophrys Hertw. et Lesser. Biol Zentralbl 47: 517–536.
- Lloyd, F. E. (1926). Some features of structure and behavior in Vampyrella lateritia. Science, 63(1631), 364-365. doi:10.1126/science.63.1631.364
When attacking, the Vampyrella organism flattens along the filament surface of its prey. Upon attachment, the long pseudopodia disappear leaving only shorter pin-like pseudopodia. A violent shock motion is usually observed as the Vampyrella attacks the algae. Within minutes cell wall digestion initiates and the vampyrellid begins to swell as the contents of the algae are drained. The cell wall begins to bend inward due to a loss in turgor pressure causing adjacent cells of the algae to have greater pressure. Upon fully digesting through the cell wall, a hole is created in the cell wall of the algae. The vampyrellid swells rapidly and the prey cell disarticulates. The protoplast of the prey is then sucked out into a food vacuole. Remaining contents are then drawn out using pseudopodia. The vampyrellid uses its pseudopodia to move from cell to cell repeating the process. Excess water absorbed from prey cells is removed by numerous small contractile vacuoles on the periphery of the organism, maintaining an only slightly increased size after each meal. Vampyrella have been observed to both perforate the cell wall on the cell body as well as breaking the joints between algal cells to acquire the contents of the cell.
- West, G. S. (1901). On some british freshwater rhizopods and heliozoa. Journal of the Linnean Society of London, Zoology, 28(183), 308-342. doi:10.1111/j.1096-3642.1901.tb01754.x
- Hoogenraad HR (1927) Bemerkungen über das Genus Leptophrys Hertw. et Lesser. Biol Zentralbl 47: 517–536.
- Lloyd, F. E. (1926). Some features of structure and behavior in Vampyrella lateritia. Science, 63(1631), 364-365. doi:10.1126/science.63.1631.364
Vampyrella have also shown to be selective of food type. Once in contact with filaments, it brings its body toward the filament. If the algae are rejected it will move on.Among this selectivity, there is also differences among species within the genus. This has been observed as Vampyrella lateritia refuse to eat Oedogonium while it will be eaten by Vampyrella pendula.
- Hess, S., Sausen, N., & Melkonian, M. (2012). Shedding light on vampires: The phylogeny of vampyrellid amoebae revisited. PLOS One, 7(2), e31165. doi:10.1371/journal.pone.0031165
- Timpano, P., & Pfiester, L. A. (1986). Observations on “Vampyrella penula-Stylodinium sphaera” and the ultrastructure of the reproductive cyst. American Journal of Botany, 73(9), 1341-1350. doi:10.2307/2444068
- Lloyd, F. E. (1926). Some features of structure and behavior in Vampyrella lateritia. Science, 63(1631), 364-365. doi:10.1126/science.63.1631.364
Ecology
Vampyrella are known to be algivorous predators which can be detrimental to the biomass of microalgal cultures. Some green microalgae are capable of rapid growth and synthesize large quantities of protein, starch and lipid. In addition, microalgae can be used in carbon capture, animal feeding, waste water bioremediation and biofuels. The main cause in loss of microalgal culture biomass is microbial contamination.
- Gong, Y., Patterson, D. J., Li, Y., Hu, Z., Sommerfeld, M., Chen, Y., & Hu, Q. (2015). Vernalophrys algivore gen. nov., sp. nov. (rhizaria: Cercozoa: Vampyrellida), a new algal predator isolated from outdoor mass culture of scenedesmus dimorphus. Applied and Environmental Microbiology, 81(12), 3900-3913. doi:10.1128/AEM.00160-15
List of species
- Vampyrella closterii
- Vampyrella incolor
- Vampyrella inermis
- Vampyrella lateritia
- Vampyrella multiformis
- Vampyrella pedata
- Vampyrella pendula
- Vampyrella ulothricus
- Vampyrella variabilis
- Vampyrella velata
Vampyrella lateritia is a freshwater species of predatory amoebae that feeds on species of algae and is known for its specialized feeding strategy of removing, digesting, and ingesting the cellular contents of its prey. It is the type species of the genus Vampyrella and has been identified in numerous locations around the world including Brazil, Germany, and the eastern United States. Along with Vampyrella pendula, its genome was sequenced in 2012.
- Hess, Sebastian; Suthaus, Andreas (2022-02-01). “The Vampyrellid Amoebae (Vampyrellida, Rhizaria)”. Protist. 173 (1): 125854. doi:10.1016/j.protis.2021.125854. ISSN 1434-4610. PMID 35091168. S2CID 245303468.
- “Vampyrella Morphology”. www.nies.go.jp. Retrieved 2022-05-25.
- Hardoim, Edna Lopes; Heckman, Charles W. (1996). “The Seasonal Succession of Biotic Communities in Wetlands of the Tropical Wet-and-Dry Climatic Zone: IV. The Free-Living Sarcodines and Ciliates of the Pantanal of Mato Grosso, Brazil”. Internationale Revue der gesamten Hydrobiologie und Hydrographie (in German). 81 (3): 367–384. doi:10.1002/iroh.19960810307.
- Hess, Sebastian; Sausen, Nicole; Melkonian, Michael (2012-02-15). “Shedding Light on Vampires: The Phylogeny of Vampyrellid Amoebae Revisited”. PLOS ONE. 7 (2): e31165. doi:10.1371/journal.pone.0031165. ISSN 1932-6203. PMC 3280292. PMID 22355342.
- Berney, Cédric; Romac, Sarah; Mahé, Frédéric; Santini, Sébastien; Siano, Raffaele; Bass, David (December 2013). “Vampires in the oceans: predatory cercozoan amoebae in marine habitats”. The ISME Journal. 7 (12): 2387–2399. doi:10.1038/ismej.2013.116. ISSN 1751-7370. PMC 3834849. PMID 23864128.
Life cycle
Vampyrella lateritia has four life stages that revolve about the feeding cycle: motile trophozoites (the activated, feeding stage), plasmodia in which the cytoplasm contains many nuclei, digestive cysts, and resting cysts. It has been observed feeding on species from the genera Zygnema, Spirogyra, and Mougeotia and is considered a specialist predator as its known prey is restricted to a limited number of green algal species.
- Hess, Sebastian; Sausen, Nicole; Melkonian, Michael (2012-02-15). “Shedding Light on Vampires: The Phylogeny of Vampyrellid Amoebae Revisited”. PLOS ONE. 7 (2): e31165. doi:10.1371/journal.pone.0031165. ISSN 1932-6203. PMC 3280292. PMID 22355342.
- West, G. S. (1901). “On some British Freshwater Rhizopods and Heliozoa”. Journal of the Linnean Society of London, Zoology. 28 (183): 308–342. doi:10.1111/j.1096-3642.1901.tb01754.x.
- Hülsmann, Norbert; Grębecki, Andrzej (1995-05-26). “Induction of lobopodia and lamellipodia in a filopodial organism (Vampyrella lateritia)”. European Journal of Protistology. 31 (2): 182–189. doi:10.1016/S0932-4739(11)80442-6. ISSN 0932-4739.
Like other vampyrellids, Vampyrella lateritia grows well between 10°C and room temperature. It contains intracellular bacteria that have not yet been conclusively identified, although the morphology of the endosymbiotic bacteria resembles Ca. Megaira polyxenopila, a species of bacteria in the family Rickettsiaceae.
- Hausmann, K (1978). “(Particules du Type Bacterie et Virus Dans le Cytoplasme du RHizopode V. L)La Lateritia”. Ann. Stu. Biol. Bess.-En-Chandesse (in English and French). 11: 102–188.
- Hess, Sebastian (2017-02-01). “Description of Hyalodiscus flabellus sp. nov. (Vampyrellida, Rhizaria) and Identification of its Bacterial Endosymbiont, “Candidatus Megaira polyxenophila” (Rickettsiales, Alphaproteobacteria)”. Protist. 168 (1): 109–133. doi:10.1016/j.protis.2016.11.003. ISSN 1434-4610. PMID 28064061.
Trophozoites
In this stage of action and feeding, the cells are compact and spherical with radiating filopodia and pseudopodia, moving freely through the water column. The central cell body is orange and the pseudopodia are colourless. In order to move, the filopodia are positioned under the spherical body and slowly rolls the entire cell. Along the pseudopodia, numerous granules known as membranosomes shoot rapidly out of the cell cortex, connected by a thin strand of cytoplasm, and are retracted.
- Hülsmann, Norbert; Grębecki, Andrzej (1995-05-26). “Induction of lobopodia and lamellipodia in a filopodial organism (Vampyrella lateritia)”. European Journal of Protistology. 31 (2): 182–189. doi:10.1016/S0932-4739(11)80442-6. ISSN 0932-4739.
- Hess, Sebastian (2017-02-01). “Description of Hyalodiscus flabellus sp. nov. (Vampyrellida, Rhizaria) and Identification of its Bacterial Endosymbiont, “Candidatus Megaira polyxenophila” (Rickettsiales, Alphaproteobacteria)”. Protist. 168 (1): 109–133. doi:10.1016/j.protis.2016.11.003. ISSN 1434-4610. PMID 28064061.
Trophozoites attach to an algal cell and retract their long pseudopodia, flattening the cell body tightly against the algae to increase the contact area. Feeding is proceeded by the dissolution of a hole 5-7 µm in diameter in the algae’s cell wall. After several minutes of this, the cell wall bursts and the exposed protoplast is engulfed into a large food vacuole. This process is known as plasmoptysis and resembles a sucking motion. It is likely the origin of the genus name Vampyrella, Latin for ‘small vampire’. The remains of the destroyed protoplast still within the algal cell are then engulfed by an ingestion pseudopodium. Vampyrella lateritia can consume several algal cells before entering the digestive cyst phase. After absorbing a single algal cell, Vampyrella lateritia is about ten times its original volume. However the dramatic increase in volume is only temporary and the amoeba returns to its normal volume within a few minutes. Vampyrella lateritia are known to only feed on live prey.
- Lloyd, Francis E. (1926-04-02). “Some Features of Structure and Behavior in Vampyrella lateritia“. Science. 63 (1631): 364–365. doi:10.1126/science.63.1631.364. ISSN 0036-8075. PMID 17819823.
- Carter, M.R.; Gregorich, E.G., eds. (2007-08-03). Soil Sampling and Methods of Analysis. Boca Raton, Fla.: CRC Press. doi:10.1201/9781420005271. ISBN 9780429126222.
- Old, K. M.; Darbyshire, J. F. (1978-01-01). “Soil fungi as food for giant amoebae”. Soil Biology and Biochemistry. 10 (2): 93–100. doi:10.1016/0038-0717(78)90077-9. ISSN 0038-0717.
Plasmodia
Individual amoeba can fuse into large and deformed plasmodia. The structure and colour are the same as the trophozoites, however this stage occurs predominantly in old cultures of Vampyrella lateritia where cell density is high and nutrients are limited. However this stage has been observed in laboratory settings, so it is unclear the conditions under which Vampyrella lateritia would form plasmodia in natural conditions.
- Hess, Sebastian; Suthaus, Andreas (2022-02-01). “The Vampyrellid Amoebae (Vampyrellida, Rhizaria)”. Protist. 173 (1): 125854. doi:10.1016/j.protis.2021.125854. ISSN 1434-4610. PMID 35091168. S2CID 245303468.
Digestive cysts
During digestive cyst formation, the trophozoite retracts its pseudopodia and secretes a cell wall. The digestive cysts have two cyst envelopes, where the inner is stronger than the outer. Unlike other vampyrellid amoebae, Vampyrella lateritia retains its separate food vacuoles throughout the entire digestive phase. The digestive phase is marked by a colour change of the cytoplasm as nutrients are digested – as a result, the colour of the digestive cyst is a good indicator for the cyst’s maturity. Vampyrella lateritia is often seen as red or orange. After digestion is complete, trophozoites hatch and leave the parent cell through holes in the cell wall created as part of the exocytosis of food remnants.
- Hess, Sebastian; Suthaus, Andreas (2022-02-01). “The Vampyrellid Amoebae (Vampyrellida, Rhizaria)”. Protist. 173 (1): 125854. doi:10.1016/j.protis.2021.125854. ISSN 1434-4610. PMID 35091168. S2CID 245303468.
- Hess, Sebastian; Sausen, Nicole; Melkonian, Michael (2012-02-15). “Shedding Light on Vampires: The Phylogeny of Vampyrellid Amoebae Revisited”. PLOS ONE. 7 (2): e31165. doi:10.1371/journal.pone.0031165. ISSN 1932-6203. PMC 3280292. PMID 22355342.
- Stokes, Alfred Cheatham (1887). Microscopy for Beginners: Or, Common Objects from the Ponds and Ditches. Harper & brothers. pp. 118–119.
Although the feeding process only lasts for several minutes in Vampyrella lateritia, the digestive process takes much longer, typically one to two days.
Resting cysts
As resting cysts, Vampyrella lateritia can survive freezing and desiccation for at least three years. Resting cysts have condensed contents and numerous cell walls, however the resting cyst stage of life is not an obligatory part of all Vampyrella lateritia life cycle, unlike the digestive cyst phase. The Vampyrella lateritia can return to the trophozoite phase in the presence of algae prey organisms and fresh medium.
- Hess, Sebastian; Suthaus, Andreas (2022-02-01). “The Vampyrellid Amoebae (Vampyrellida, Rhizaria)”. Protist. 173 (1): 125854. doi:10.1016/j.protis.2021.125854. ISSN 1434-4610. PMID 35091168. S2CID 245303468.
References
- RÖPSTORF, P., HÜLSMANN, N., & HAUSMANN, K. (1994). Comparative fine structural investigations of interphase and mitotic nuclei of vampyrellid filose amoebae. The Journal of Eukaryotic Microbiology, 41(1), 18-30. doi:10.1111/j.1550-7408.1994.tb05930.x
- Cienkowski, L. (1865). Beiträge zur kenntniss der monaden. Archiv Für Mikroskopische Anatomie, 1(1), 203–232. doi:10.1007/BF02961414
- Hess, S., Sausen, N., & Melkonian, M. (2012). Shedding light on vampires: The phylogeny of vampyrellid amoebae revisited. PLOS One, 7(2), e31165. doi:10.1371/journal.pone.0031165
- Leidy, J., Biodiversity Heritage Library, & Canadian Libraries. (1879). Fresh-water rhizopods of north America. p. 253-256.
- West, G. S. (1901). On some british freshwater rhizopods and heliozoa. Journal of the Linnean Society of London, Zoology, 28(183), 308-342. doi:10.1111/j.1096-3642.1901.tb01754.x
- Hoogenraad HR (1927) Bemerkungen über das Genus Leptophrys Hertw. et Lesser. Biol Zentralbl 47: 517–536.
- Timpano, P., & Pfiester, L. A. (1986). Observations on “Vampyrella penula-Stylodinium sphaera” and the ultrastructure of the reproductive cyst. American Journal of Botany, 73(9), 1341-1350. doi:10.2307/2444068
- Lloyd, F. E. (1926). Some features of structure and behavior in Vampyrella lateritia. Science, 63(1631), 364-365. doi:10.1126/science.63.1631.364
- Gong, Y., Patterson, D. J., Li, Y., Hu, Z., Sommerfeld, M., Chen, Y., & Hu, Q. (2015). Vernalophrys algivore gen. nov., sp. nov. (rhizaria: Cercozoa: Vampyrellida), a new algal predator isolated from outdoor mass culture of scenedesmus dimorphus. Applied and Environmental Microbiology, 81(12), 3900-3913. doi:10.1128/AEM.00160-15
- Hess, Sebastian; Suthaus, Andreas (2022-02-01). “The Vampyrellid Amoebae (Vampyrellida, Rhizaria)”. Protist. 173 (1): 125854. doi:10.1016/j.protis.2021.125854. ISSN 1434-4610. PMID 35091168. S2CID 245303468.
- “Vampyrella Morphology”. www.nies.go.jp. Retrieved 2022-05-25.
- Hardoim, Edna Lopes; Heckman, Charles W. (1996). “The Seasonal Succession of Biotic Communities in Wetlands of the Tropical Wet-and-Dry Climatic Zone: IV. The Free-Living Sarcodines and Ciliates of the Pantanal of Mato Grosso, Brazil”. Internationale Revue der gesamten Hydrobiologie und Hydrographie (in German). 81 (3): 367–384. doi:10.1002/iroh.19960810307.
- Hess, Sebastian; Sausen, Nicole; Melkonian, Michael (2012-02-15). “Shedding Light on Vampires: The Phylogeny of Vampyrellid Amoebae Revisited”. PLOS ONE. 7 (2): e31165. doi:10.1371/journal.pone.0031165. ISSN 1932-6203. PMC 3280292. PMID 22355342.
- Berney, Cédric; Romac, Sarah; Mahé, Frédéric; Santini, Sébastien; Siano, Raffaele; Bass, David (December 2013). “Vampires in the oceans: predatory cercozoan amoebae in marine habitats”. The ISME Journal. 7 (12): 2387–2399. doi:10.1038/ismej.2013.116. ISSN 1751-7370. PMC 3834849. PMID 23864128.
- Hülsmann, Norbert; Grębecki, Andrzej (1995-05-26). “Induction of lobopodia and lamellipodia in a filopodial organism (Vampyrella lateritia)”. European Journal of Protistology. 31 (2): 182–189. doi:10.1016/S0932-4739(11)80442-6. ISSN 0932-4739.
- Hausmann, K (1978). “(Particules du Type Bacterie et Virus Dans le Cytoplasme du RHizopode V. L)La Lateritia”. Ann. Stu. Biol. Bess.-En-Chandesse (in English and French). 11: 102–188.
- Hess, Sebastian (2017-02-01). “Description of Hyalodiscus flabellus sp. nov. (Vampyrellida, Rhizaria) and Identification of its Bacterial Endosymbiont, “Candidatus Megaira polyxenophila” (Rickettsiales, Alphaproteobacteria)”. Protist. 168 (1): 109–133. doi:10.1016/j.protis.2016.11.003. ISSN 1434-4610. PMID 28064061.
- Lloyd, Francis E. (1926-04-02). “Some Features of Structure and Behavior in Vampyrella lateritia“. Science. 63 (1631): 364–365. doi:10.1126/science.63.1631.364. ISSN 0036-8075. PMID 17819823.
- Carter, M.R.; Gregorich, E.G., eds. (2007-08-03). Soil Sampling and Methods of Analysis. Boca Raton, Fla.: CRC Press. doi:10.1201/9781420005271. ISBN 9780429126222.
- Old, K. M.; Darbyshire, J. F. (1978-01-01). “Soil fungi as food for giant amoebae”. Soil Biology and Biochemistry. 10 (2): 93–100. doi:10.1016/0038-0717(78)90077-9. ISSN 0038-0717.
- Stokes, Alfred Cheatham (1887). Microscopy for Beginners: Or, Common Objects from the Ponds and Ditches. Harper & brothers. pp. 118–119.


Leave a Reply