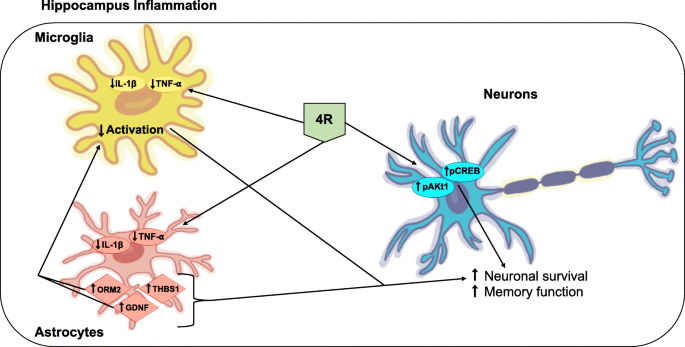
Tobacco-derived 4R-cembranoid confers neuroprotection against LPS-induced hippocampal inflammation in mice (decreases levels of pro-inflammatory cytokines; improves memory function; activates STAT3, Akt1, and CREB phosphorylation; and upregulates the mRNA levels of ORM2, GDNF, and C3) independent of the α7 nicotinic receptor
Rojas-Colón, L.A., Dash, P.K., Morales-Vías, F.A. et al. 4R-cembranoid confers neuroprotection against LPS-induced hippocampal inflammation in mice. J Neuroinflammation 18, 95 (2021). https://doi.org/10.1186/s12974-021-02136-9
LPS: Lipopolysaccharide
“4R protects the hippocampus against inflammation and memory impairments triggered by LPS by lowering TNF-α and IL-1β levels and activation of the Akt1 and CREB signaling pathways. Astrocyte proteins involved in neuronal survival also seem to be modulated by 4R. The effects of 4R in the hippocampus are independent of α7 nicotinic receptors. The diagram below represents a model of 4R action in the hippocampus during inflammation.“
Some other notes
Lipopolysaccharides (LPS) are large molecules consisting of a lipid and a polysaccharide that are bacterial toxins. They are composed of an O-antigen, an outer core, and an inner core all joined by covalent bonds, and are found in the bacterial capsule, the outermost membrane of cell envelope of Gram-negative bacteria, such as E. coli and Salmonella. Today, the term endotoxin is often used synonymously with LPS, although there are a few endotoxins (in the original sense of toxins that are inside the bacterial cell that are released when the cell disintegrates) that are not related to LPS, such as the so-called delta endotoxin proteins produced by Bacillus thuringiensis. Lipopolysaccharides can have substantial impacts on human health, primarily through interactions with the immune system. LPS is a potent activator of the immune system and pyrogen (agent that causes fever). In severe cases, LPS can play a role in causing septic shock. In lower levels and over a longer time period, there is evidence LPS may play an important and harmful role in autoimmunity, obesity, depression, and cellular senescence.
- Avila-Calderón ED, Ruiz-Palma MD, Aguilera-Arreola MG, Velázquez-Guadarrama N, Ruiz EA, Gomez-Lunar Z, et al. (2021). “Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis”. Frontiers in Microbiology. 12: 557902. doi:10.3389/fmicb.2021.557902. PMC 7969528. PMID 33746909.
- Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, et al. (February 1994). “Bacterial endotoxin: molecular relationships of structure to activity and function”. FASEB Journal. 8 (2): 217–225. doi:10.1096/fasebj.8.2.8119492. PMID 8119492. S2CID 28156137.
- Höfte H, de Greve H, Seurinck J, Jansens S, Mahillon J, Ampe C, et al. (December 1986). “Structural and functional analysis of a cloned delta endotoxin of Bacillus thuringiensis berliner 1715”. European Journal of Biochemistry. 161 (2): 273–280. doi:10.1111/j.1432-1033.1986.tb10443.x. PMID 3023091.
- Roth J, Blatteis CM (October 2014). “Mechanisms of fever production and lysis: lessons from experimental LPS fever”. Comprehensive Physiology. 4 (4): 1563–1604. doi:10.1002/cphy.c130033. ISBN 978-0-470-65071-4. PMID 25428854.
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. (February 2013). “Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012”. Critical Care Medicine. 41 (2): 580–637. doi:10.1097/CCM.0b013e31827e83af. PMID 23353941. S2CID 34855187.
- Moran AP, Prendergast MM, Appelmelk BJ (December 1996). “Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease”. FEMS Immunology and Medical Microbiology. 16 (2): 105–115. doi:10.1016/s0928-8244(96)00072-7. PMID 8988391.
- Moreno-Navarrete JM, Ortega F, Serino M, Luche E, Waget A, Pardo G, et al. (November 2012). “Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance”. International Journal of Obesity. 36 (11): 1442–1449. doi:10.1038/ijo.2011.256. PMID 22184060.
- Lasselin J, Schedlowski M, Karshikoff B, Engler H, Lekander M, Konsman JP (August 2020). “Comparison of bacterial lipopolysaccharide-induced sickness behavior in rodents and humans: Relevance for symptoms of anxiety and depression”. Neuroscience and Biobehavioral Reviews. 115: 15–24. doi:10.1016/j.neubiorev.2020.05.001. PMID 32433924. S2CID 218665128.
- Wei W, Ji S (December 2018). “Cellular senescence: Molecular mechanisms and pathogenicity”. Journal of Cellular Physiology. 233 (12): 9121–9135. doi:10.1002/jcp.26956. PMID 30078211. S2CID 51924586.
Lipopolysaccharides are frequent contaminants in plasmid DNA prepared from bacteria or proteins expressed from bacteria, and must be removed from the DNA or protein to avoid contaminating experiments and to avoid toxicity of products manufactured using industrial fermentation.
- Wicks IP, Howell ML, Hancock T, Kohsaka H, Olee T, Carson DA (March 1995). “Bacterial lipopolysaccharide copurifies with plasmid DNA: implications for animal models and human gene therapy”. Human Gene Therapy. 6 (3): 317–323. doi:10.1089/hum.1995.6.3-317. PMID 7779915.
Ovalbumin is frequently contaminated with endotoxins. Ovalbumin is one of the extensively studied proteins in animal models and also an established model allergen for airway hyper-responsiveness (AHR). Commercially available ovalbumin that is contaminated with LPS can falsify research results, as it does not accurately reflect the effect of the protein antigen on animal physiology.
- Watanabe J, Miyazaki Y, Zimmerman GA, Albertine KH, McIntyre TM (October 2003). “Endotoxin contamination of ovalbumin suppresses murine immunologic responses and development of airway hyper-reactivity”. The Journal of Biological Chemistry. 278 (43): 42361–42368. doi:10.1074/jbc.M307752200. PMID 12909619.
In pharmaceutical production, it is necessary to remove all traces of endotoxin from drug product containers, as even small amounts of endotoxin will cause illness in humans. A depyrogenation oven is used for this purpose. Temperatures in excess of 300 °C are required to fully break down LPS.
- Komski L (16 December 2014). “The Detection of Endotoxins Via the LAL Test, the Chromogenic Method”. Wako Chemicals USA, Inc. Archived from the original on 29 March 2015. Retrieved 14 March 2015.
The standard assay for detecting presence of endotoxin is the Limulus Amebocyte Lysate (LAL) assay, utilizing blood from the Horseshoe crab (Limulus polyphemus).
- Iwanaga S (May 2007). “Biochemical principle of Limulus test for detecting bacterial endotoxins”. Proceedings of the Japan Academy. Series B, Physical and Biological Sciences. 83 (4): 110–119. Bibcode:2007PJAB…83..110I. doi:10.2183/pjab.83.110. PMC 3756735. PMID 24019589.
Very low levels of LPS can cause coagulation of the limulus lysate due to a powerful amplification through an enzymatic cascade. However, due to the dwindling population of horseshoe crabs, and the fact that there are factors that interfere with the LAL assay, efforts have been made to develop alternative assays, with the most promising ones being ELISA tests using a recombinant version of a protein in the LAL assay, Factor C.
- Ding JL, Ho B (August 2001). “A new era in pyrogen testing” (PDF). Trends in Biotechnology. 19 (8): 277–281. doi:10.1016/s0167-7799(01)01694-8. PMID 11451451. Archived from the original (PDF) on 2 January 2014. Retrieved 2 January 2014.
Lipopolysaccharide, is a significant factor that makes bacteria harmful, and it helps categorize them into different groups based on their structure and function. This makes LPS a useful marker for telling apart various Gram-negative bacteria. Swiftly identifying and understanding the types of pathogens involved is crucial for promptly managing and treating infections. Since LPS is the main trigger for the immune response in our cells, it acts as an early signal of an acute infection. Therefore, LPS testing is more specific and meaningful than many other serological tests. The current methods for testing LPS are quite sensitive, but many of them struggle to differentiate between different LPS groups. Additionally, the nature of LPS, which has both water-attracting and water-repelling properties (amphiphilic), makes it challenging to develop sensitive and user-friendly tests. The typical detection methods rely on identifying the lipid A part of LPS. However, this method has limitations because Lipid A is very similar among different bacterial species and serotypes. LPS testing techniques fall into six categories, and they often overlap: in vivo tests, in vitro tests, modified immunoassays, biological assays, and chemical assays.
- Page MJ, Kell DB, Pretorius E (2022). “The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation”. Chronic Stress. 6: 24705470221076390. doi:10.1177/24705470221076390. PMC 8829728. PMID 35155966.
LPS is a powerful toxin that, when in the body, triggers inflammation by binding to cell receptors. Excessive LPS in the blood can lead to endotoxemia, potentially causing a harmful condition called septic shock. This condition includes symptoms like rapid heart rate, quick breathing, temperature changes, and blood clotting issues, resulting in blood vessels widening and reduced blood volume, leading to cellular dysfunction. Recent research indicates that even small LPS exposure is associated with autoimmune diseases and allergies. High levels of LPS in the blood can lead to metabolic syndrome, increasing the risk of conditions like diabetes, heart disease, and liver problems. LPS also plays a crucial role in symptoms caused by infections from harmful bacteria, including severe conditions like Waterhouse-Friderichsen syndrome, meningococcemia, and meningitis. Certain bacteria can adapt their LPS to cause long-lasting infections in the respiratory and digestive systems. Recent studies have shown that LPS disrupts cell membrane lipids, affecting cholesterol and metabolism, potentially leading to high cholesterol, abnormal blood lipid levels, and non-alcoholic fatty liver disease. In some cases, LPS can interfere with toxin clearance, which may be linked to neurological issues.
- Page MJ, Kell DB, Pretorius E (2022). “The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation”. Chronic Stress. 6: 24705470221076390. doi:10.1177/24705470221076390. PMC 8829728. PMID 35155966.
See also
- Bioaerosol
- Depyrogenation
- Host-pathogen interface
- Mucopolysaccharide
- Nesfatin-1
- Schwartzman reaction
- AOAH
STAT3
Niclosamide seems to inhibit the STAT3 signalling pathway.
- Ren X, Duan L, He Q, Zhang Z, Zhou Y, Wu D, Pan J, Pei D, Ding K (2010). “Identification of Niclosamide as a New Small-Molecule Inhibitor of the STAT3 Signaling Pathway”. ACS Medicinal Chemistry Letters. 1 (9): 454–9. doi:10.1021/ml100146z. PMC 4007964. PMID 24900231.
Nicotinamide (a type of Vitamin B3 – the most common and very worst type IMO) naturally inhibits STAT3.
- Wang W, Hu Y, Yang C, Zhu S, Wang X, Zhang Z, et al. (2018). “Decreased NAD Activates STAT3 and Integrin Pathways to Drive Epithelial-Mesenchymal Transition”. Mol Cell Proteomics. 17 (10): 2005–2017. doi:10.1074/mcp.RA118.000882. PMC 6166677. PMID 29980616.
However NAC (Acetylcysteine) inhibits STAT3 inhibitors.
That’s very interesting to me because according to the internet, NAC (which has been available as a supplement is currently being hijacked by all manner of cretins) is the antidote to paracetamol/tylenol poisoning and maybe aspartame poisoning (which is apparently more common than they care to admit particularly in the nonsmoker as the nonsmoker does not have a human metabolism or clearance system)
- Uchihara Y, Ohe T, Mashino T, Kidokoro T, Tago K, Tamura H, et al. (2019). “N-Acetyl cysteine prevents activities of STAT3 inhibitors, Stattic and BP-1-102 independently of its antioxidant properties”. Pharmacol Rep. 71 (6): 1067–1078. doi:10.1016/j.pharep.2019.05.021. PMID 31627175. S2CID 210983316.
STAT3 has been shown to interact with:
- AR
- ELP2
- EP300
- EGFR
- HIF1A
- JAK1
- JUN
- KHDRBS1
- mTOR
- MYOD1
- NDUFA13
- NFKB1
- NR3C1
- NCOA1
- PML
- RAC1
- RELA
- RET
- RPA2
- STAT1
- Stathmin
- Src
- TRIP10
- KPNA4
Akt1
RAC(Rho family)-alpha serine/threonine-protein kinase is an enzyme that in humans is encoded by the AKT1 gene. This enzyme belongs to the AKT subfamily of serine/threonine kinases that contain SH2 (Src homology 2-like) protein domains. It is commonly referred to as PKB, or by both names as “Akt/PKB”.
The serine-threonine protein kinase AKT1 is catalytically inactive in serum-starved primary and immortalized fibroblasts. AKT1 and the related AKT2 are activated by platelet-derived growth factor. The activation is rapid and specific, and it is abrogated by mutations in the pleckstrin homology domain of AKT1. It was shown that the activation occurs through phosphatidylinositol 3-kinase. In the developing nervous system AKT is a critical mediator of growth factor-induced neuronal survival. Survival factors can suppress apoptosis in a transcription-independent manner by activating the serine/threonine kinase AKT1, which then phosphorylates and inactivates components of the apoptotic machinery. Mice lacking Akt1 display a 25% reduction in body mass, indicating that Akt1 is critical for transmitting growth-promoting signals, most likely via the IGF1 receptor. Mice lacking Akt1 are also resistant to cancer: They experience considerable delay in tumor growth initiated by the large T antigen or the Neu oncogene.
A single-nucleotide polymorphism in this gene causes Proteus syndrome. In 2011, a mutation in AKT1 was strongly associated with Proteus syndrome, the disease that probably affected the Elephant Man.
- Lindhurst MJ, Sapp JC, Teer JK, Johnston JJ, Finn EM, Peters K, Turner J, Cannons JL, Bick D, Blakemore L, Blumhorst C, Brockmann K, Calder P, Cherman N, Deardorff MA, Everman DB, Golas G, Greenstein RM, Kato BM, Keppler-Noreuil KM, Kuznetsov SA, Miyamoto RT, Newman K, Ng D, O’Brien K, Rothenberg S, Schwartzentruber DJ, Singhal V, Tirabosco R, Upton J, Wientroub S, Zackai EH, Hoag K, Whitewood-Neal T, Robey PG, Schwartzberg PL, Darling TN, Tosi LL, Mullikin JC, Biesecker LG (2011). “A mosaic activating mutation in AKT1 associated with the Proteus syndrome”. N. Engl. J. Med. 365 (7): 611–9. doi:10.1056/NEJMoa1104017. PMC 3170413. PMID 21793738.
- Cohen MM (2014). “Proteus syndrome review: molecular, clinical, and pathologic features”. Clin. Genet. 85 (2): 111–9. doi:10.1111/cge.12266. PMID 23992099. S2CID 204999819.
- Lindhurst MJ, Sapp JC, Teer JK, Johnston JJ, Finn EM, Peters K, Turner J, Cannons JL, Bick D, Blakemore L, Blumhorst C, Brockmann K, Calder P, Cherman N, Deardorff MA, Everman DB, Golas G, Greenstein RM, Kato BM, Keppler-Noreuil KM, Kuznetsov SA, Miyamoto RT, Newman K, Ng D, O’Brien K, Rothenberg S, Schwartzentruber DJ, Singhal V, Tirabosco R, Upton J, Wientroub S, Zackai EH, Hoag K, Whitewood-Neal T, Robey PG, Schwartzberg PL, Darling TN, Tosi LL, Mullikin JC, Biesecker LG (27 July 2011). “A Mosaic Activating Mutation in Associated with the Proteus Syndrome”. New England Journal of Medicine. 365 (7): 611–619. doi:10.1056/NEJMoa1104017. PMC 3170413. PMID 21793738
AKT (now also called AKT1) was originally identified as the oncogene in the transforming retrovirus, AKT8. The name Akt stands for Ak strain transforming. The origins of the Akt name date back to 1928,
- Staal SP, Hartley JW, Rowe WP (July 1977). “Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma”. Proc. Natl. Acad. Sci. U.S.A. 74 (7): 3065–7. Bibcode:1977PNAS…74.3065S. doi:10.1073/pnas.74.7.3065. PMC 431413. PMID 197531.
- Staal SP (July 1987). “Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma”. Proc. Natl. Acad. Sci. U.S.A. 84 (14): 5034–7. Bibcode:1987PNAS…84.5034S. doi:10.1073/pnas.84.14.5034. PMC 305241. PMID 3037531.
AKT1 has been shown to interact with:
- AKTIP
- BRAF
- BRCA1
- C-Raf,
- CDKN1B
- CHUK
- GAB2
- HSP90AA1
- ILK
- KRT10
- MAP2K4
- MAP3K11
- MAP3K8
- MAPK14
- MAPKAPK2
- MARK2
- MTCP1
- MTOR
- NPM1
- NR4A1
- NR3C4
- PKN2
- PRKCQ
- PDPK1
- PLXNA1
- TCL1A
- TRIB3
- TSC1
- TSC2
- YWHAZ
See also
- AKT – the AKT family of proteins
- AKT2 – the gene for the second member of the AKT family
- AKT3 – the gene for the third member of the AKT family
- Proteus syndrome
CREB phosphorylation
Not to be confused with Clean Renewable Energy Bonds.
CREB-TF (CREB, cAMP response element-binding protein) is a cellular transcription factor. It binds to certain DNA sequences called cAMP response elements (CRE), thereby increasing or decreasing the transcription of the genes. CREB was first described in 1987 as a cAMP-responsive transcription factor regulating the somatostatin gene. Genes whose transcription is regulated by CREB include: c-fos, BDNF, tyrosine hydroxylase, numerous neuropeptides (such as somatostatin, enkephalin, VGF, corticotropin-releasing hormone), and genes involved in the mammalian circadian clock (PER1, PER2). CREB is closely related in structure and function to CREM (cAMP response element modulator) and ATF-1 (activating transcription factor-1) proteins. CREB proteins are expressed in many animals, including humans. CREB has a well-documented role in neuronal plasticity and long-term memory formation in the brain and has been shown to be integral in the formation of spatial memory. CREB downregulation is implicated in the pathology of Alzheimer’s disease and increasing the expression of CREB is being considered as a possible therapeutic target for Alzheimer’s disease. CREB also has a role in photoentrainment in mammals.
- Bourtchuladze; et al. (1994). “Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein”. Cell. 79 (1): 59–68. doi:10.1016/0092-8674(94)90400-6. PMID 7923378. S2CID 17250247.
- Purves, Dale; George J. Augustine; David Fitzpatrick; William C. Hall; Anthony-Samuel LaMantia; James O. McNamara & Leonard E. White (2008). Neuroscience (4th ed.). Sinauer Associates. pp. 170–6. ISBN 978-0-87893-697-7.
- Montminy, MR; Bilezikjian, LM (1987). “Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene”. Nature. 328 (6126): 175–178. Bibcode:1987Natur.328..175M. doi:10.1038/328175a0. PMID 2885756. S2CID 4345292.
- Dibner, Charna; Schibler, Ueli; Albrecht, Urs (2010). “The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks” (PDF). Annual Review of Physiology. 72 (1): 517–549. doi:10.1146/annurev-physiol-021909-135821. PMID 20148687.
- Silva; et al. (1998). “CREB and Memory” (PDF). Annual Review of Neuroscience. 21: 127–148. doi:10.1146/annurev.neuro.21.1.127. PMID 9530494. Archived from the original (PDF) on 28 August 2008. Retrieved 22 January 2010.
- Downregulation of CREB expression in Alzheimer’s brain and in Ab-treated rat hippocampal neurons
See also
ORM2 (this doesn’t have a Wikipedia page. It is briefly mentioned on several others including ORM)
Orosomucoid (ORM) or alpha-1-acid glycoprotein (α1AGp, AGP or AAG) is an acute phase protein found in plasma. It is an alpha-globulinglycoprotein and is modulated by two polymorphic genes. It is synthesized primarily in hepatocytes and has a normal plasma concentration between 0.6–1.2 mg/mL (1–3% plasma protein). Plasma levels are affected by pregnancy, burns, certain drugs, and certain diseases, particularly HIV.
- Logan, Carolynn M.; Rice, M. Katherine (1987). Logan’s Medical and Scientific Abbreviations. Philadelphia: J. B. Lippincott Company. p. 3. ISBN 0-397-54589-4.
- Colombo S, Buclin T, Décosterd LA, Telenti A, Furrer H, Lee BL, Biollaz J, Eap CB (October 2006). “Orosomucoid (alpha1-acid glycoprotein) plasma concentration and genetic variants: effects on human immunodeficiency virus protease inhibitor clearance and cellular accumulation”. Clinical Pharmacology and Therapeutics. 80 (4): 307–18. doi:10.1016/j.clpt.2006.06.006. PMID 17015049. S2CID 684478.
See also
Lipocalins mentions ORM2 as one of the human proteins that contain lipocalin domain
The lipocalins are a family of proteins which transport small hydrophobic molecules such as steroids, bilins, retinoids, and lipids, and most lipocalins are also able to bind to complexed iron (via siderophores or flavonoids) as well as heme. They share limited regions of sequence homology and a common tertiary structure architecture. This is an eight stranded antiparallel beta barrel with a repeated + 1 topology enclosing an internal ligand binding site. These proteins are found in gram negative bacteria, vertebrate cells, and invertebrate cells, and in plants. Lipocalins have been associated with many biological processes, among them immune response, pheromone transport, biological prostaglandin synthesis, retinoid binding, and cancer cell interactions.
- Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK (November 2002). “The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition”. Molecular Cell. 10 (5): 1033–1043. doi:10.1016/s1097-2765(02)00708-6. PMID 12453412.
- Roth-Walter F, Pacios LF, Bianchini R, Jensen-Jarolim E (December 2017). “Linking iron-deficiency with allergy: role of molecular allergens and the microbiome”. Metallomics. 9 (12): 1676–1692. doi:10.1039/c7mt00241f. PMID 29120476.
- Matz JM, Drepper B, Blum TB, van Genderen E, Burrell A, Martin P, et al. (July 2020). “A lipocalin mediates unidirectional heme biomineralization in malaria parasites”. Proceedings of the National Academy of Sciences of the United States of America. 117 (28): 16546–16556. Bibcode:2020PNAS..11716546M. doi:10.1073/pnas.2001153117. PMC 7368307. PMID 32601225.
- Pervaiz S, Brew K (September 1987). “Homology and structure-function correlations between alpha 1-acid glycoprotein and serum retinol-binding protein and its relatives”. FASEB Journal. 1 (3): 209–214. doi:10.1096/fasebj.1.3.3622999. PMID 3622999. S2CID 12416282.
- Igarashi M, Nagata A, Toh H, Urade Y, Hayaishi O (June 1992). “Structural organization of the gene for prostaglandin D synthase in the rat brain”. Proceedings of the National Academy of Sciences of the United States of America. 89 (12): 5376–5380. Bibcode:1992PNAS…89.5376I. doi:10.1073/pnas.89.12.5376. PMC 49294. PMID 1608945.
- Cowan SW, Newcomer ME, Jones TA (1990). “Crystallographic refinement of human serum retinol binding protein at 2A resolution”. Proteins. 8 (1): 44–61. doi:10.1002/prot.340080108. PMID 2217163. S2CID 21613341.
- Flower DR, North AC, Attwood TK (May 1993). “Structure and sequence relationships in the lipocalins and related proteins”. Protein Science. 2 (5): 753–761. doi:10.1002/pro.5560020507. PMC 2142497. PMID 7684291.
- Godovac-Zimmermann J (February 1988). “The structural motif of beta-lactoglobulin and retinol-binding protein: a basic framework for binding and transport of small hydrophobic molecules?”. Trends in Biochemical Sciences. 13 (2): 64–66. doi:10.1016/0968-0004(88)90031-X. PMID 3238752.
- Araos P, Amador CA (2022). “Neutrophil gelatinase-associated lipocalin as an immunomodulator in endocrine hypertension”. Front Endocrinol (Lausanne). 13: 1006790. doi:10.3389/fendo.2022.1006790. PMC 9640732. PMID 36387895.
The name “lipocalin” has been proposed for this protein family, but cytosolic fatty acid binding proteins are also included. The sequences of most members of the family, the core or kernel lipocalins, are characterised by three short conserved stretches of residues, while others, the outlier lipocalin group, share only one or two of these. Proteins known to belong to this family include alpha-1-microglobulin (protein HC); major urinary proteins; alpha-1-acid glycoprotein (orosomucoid); aphrodisin; apolipoprotein D; beta-lactoglobulin; complement component C8 gamma chain; crustacyanin; epididymal-retinoic acid binding protein (E-RABP); insectacyanin; odorant binding protein (OBP); human pregnancy-associated endometrial alpha-2 globulin (PAEP); probasin (PB), a prostatic protein; prostaglandin D synthase; purpurin; Von Ebner’s gland protein (VEGP); and lizard epididymal secretory protein IV (LESP IV).
- Flower DR, North AC, Attwood TK (October 1991). “Mouse oncogene protein 24p3 is a member of the lipocalin protein family”. Biochemical and Biophysical Research Communications. 180 (1): 69–74. doi:10.1016/S0006-291X(05)81256-2. PMID 1834059.
- Kremer JM, Wilting J, Janssen LH (March 1988). “Drug binding to human alpha-1-acid glycoprotein in health and disease”. Pharmacological Reviews. 40 (1): 1–47. PMID 3064105.
- Haefliger JA, Peitsch MC, Jenne DE, Tschopp J (1991). “Structural and functional characterization of complement C8 gamma, a member of the lipocalin protein family”. Molecular Immunology. 28 (1–2): 123–131. doi:10.1016/0161-5890(91)90095-2. PMID 1707134.
- Keen JN, Caceres I, Eliopoulos EE, Zagalsky PF, Findlay JB (April 1991). “Complete sequence and model for the A2 subunit of the carotenoid pigment complex, crustacyanin”. European Journal of Biochemistry. 197 (2): 407–417. doi:10.1111/j.1432-1033.1991.tb15925.x. PMID 2026162.
- Newcomer ME (September 1993). “Structure of the epididymal retinoic acid binding protein at 2.1 A resolution”. Structure. 1 (1): 7–18. doi:10.1016/0969-2126(93)90004-Z. PMID 8069623.
- Peitsch MC, Boguski MS (October 1991). “The first lipocalin with enzymatic activity”. Trends in Biochemical Sciences. 16 (10): 363. doi:10.1016/0968-0004(91)90149-P. PMID 1723819.
- Kock K, Ahlers C, Schmale H (May 1994). “Structural organization of the genes for rat von Ebner’s gland proteins 1 and 2 reveals their close relationship to lipocalins”. European Journal of Biochemistry. 221 (3): 905–916. doi:10.1111/j.1432-1033.1994.tb18806.x. PMID 7514123.
- Morel L, Dufaure JP, Depeiges A (May 1993). “LESP, an androgen-regulated lizard epididymal secretory protein family identified as a new member of the lipocalin superfamily”. The Journal of Biological Chemistry. 268 (14): 10274–10281. doi:10.1016/S0021-9258(18)82200-1. PMID 8486691.
Human proteins that contain lipocalin domain include:
- AMBP, APOD
- C8G, CRABP1, CRABP2
- FABP1, FABP2, FABP3, FABP4, FABP5, FABP6, FABP7
- LCN1, LCN2, LCN8, LCN9, LCN10, LCN12
- OBP2A, OBP2B
- ORM1, ORM2
- PAEP, PERF15, PMP2, PTGDS
- RBP1, RBP2, RBP4, RBP5, RBP7
- UNQ2541
See also
Anticalin proteins are artificial proteins that are able to bind to antigens, either to proteins or to small molecules. They are not structurally related to antibodies, which makes them a type of antibody mimetic. Instead, they are derived from human lipocalins which are a family of naturally binding proteins. Anticalin proteins are being used in lieu of monoclonal antibodies, but are about eight times smaller with a size of about 180 amino acids and a mass of about 20 kDa. The Anticalin technology is exclusively commercialized by Pieris Pharmaceuticals in Freising, Germany. Anticalin is a registered trademark of Pieris.[citation needed] Anticalin proteins have better tissue penetration than antibodies and are stable at temperatures up to 70 °C. Unlike antibodies, they can be produced in bacterial cells like E. coli in large amounts. While antibodies can only be directed at macromolecules such as proteins and at small molecules (haptens) only if bound to macromolecules, Anticalin proteins are able to selectively bind to small molecules as well.[citation needed] They were mainly developed at the Technical University of Munich and are currently used as research tools. Diagnostic and therapeutic applications, including the use for targeted drug delivery, are being aimed at. The underlying technology was nominated for the German Future Prize in 2004. Characteristic for Anticalin proteins is their barrel structure formed by eight antiparallel β-strands pairwise connected by loops and an attached α-helix. The main structure of Anticalin proteins is identical to wild type lipocalins. Conformational deviations are primarily located in the four loops reaching in the ligand binding site. Mutagenesis of amino acids at the binding site allows for changing the affinity and selectivity.[citation needed]
- “Pieris Pharmaceuticals, Inc”. Pieris Pharmaceuticals, Inc. Retrieved 16 June 2015.
- Skerra A (June 2008). “Alternative binding proteins: anticalins – harnessing the structural plasticity of the lipocalin ligand pocket to engineer novel binding activities”. FEBS J. 275 (11): 2677–83. doi:10.1111/j.1742-4658.2008.06439.x. PMID 18435758. S2CID 19992238.
- Mutschler, Ernst; Schäfer-Korting, Monika (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. pp. 911f. ISBN 3-8047-1763-2.
- Skerra, A (2002). “Anticaline” (PDF). BIOforum (in German). Darmstadt: GIT Verlag. 4/2002: 227–229.[permanent dead link]
- “Deutscher Zukunftspreis 2004: Anticaline – Biopharmazeutische Wirkstoffe durch Protein-Design” [German Future Prize 2004: Anticalins – Biopharmaceutical agents by protein design] (in German). Stifterverband für die Deutsche Wissenschaft. Archived from the original on 8 December 2010. Retrieved 6 December 2010.
| Engineered monoclonal antibodies and antibody mimetics |
|---|
GDNF
Glial cell line-derived neurotrophic factor (GDNF) is a protein that, in humans, is encoded by the GDNFgene. GDNF is a small protein that potently promotes the survival of many types of neurons. It signals through GFRα receptors, particularly GFRα1. It is also responsible for the determination of spermatogonia into primary spermatocytes, i.e. it is received by RET proto-oncogene (RET) and by forming gradient with SCF it divides the spermatogonia into two cells. As the result there is retention of spermatogonia and formation of spermatocyte. GDNF was discovered in 1991, and is the first member of the GDNF family of ligands (GFL) found. GDNF is highly distributed throughout both the peripheral and central nervous system. It can be secreted by astrocytes, oligodendrocytes, Schwann cells, motor neurons, and skeletal muscle during the development and growth of neurons and other peripheral cells. The GDNF gene encodes a highly conserved neurotrophic factor. The recombinant form of this protein was shown to promote the survival and differentiation of dopaminergic neurons in culture, and was able to prevent apoptosis of motor neurons induced by axotomy.
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (May 1993). “GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons”. Science. 260 (5111): 1130–2. Bibcode:1993Sci…260.1130L. doi:10.1126/science.8493557. PMID 8493557.
- “Entrez Gene: GDNF glial cell derived neurotrophic factor”.
- Scott F. Gilbert
- Vastag B (August 2010). “Biotechnology: Crossing the barrier”. Nature. 466 (7309): 916–8. doi:10.1038/466916a. PMID 20725015.
- Cintrón-Colón AF, Almeida-Alves G, Boynton AM, Spitsbergen JM (October 2020). “GDNF synthesis, signaling, and retrograde transport in motor neurons”. Cell and Tissue Research. 382 (1): 47–56. doi:10.1007/s00441-020-03287-6. PMC 7529617. PMID 32897420.
C3
Complement component 3, often simply called C3, is a protein of the immune system that is found primarily in the blood. It plays a central role in the complement system of vertebrate animals and contributes to innate immunity. In humans it is encoded on chromosome 19 by a gene called C3. Deficiencies and defects of C3 result in the affected person being immunocompromised and particularly vulnerable to bacterial infections. In humans, C3 is predominantly synthesised by liver hepatocytes and to some degree by epidermis keratinocytes. Factor H is the primary regulator of C3. Deficiency of Factor H may lead to uncontrolled C3 activity through the alternative pathway of the complement system.
- de Bruijn MH, Fey GH (Feb 1985). “Human complement component C3: cDNA coding sequence and derived primary structure”. Proceedings of the National Academy of Sciences of the United States of America. 82 (3): 708–12. Bibcode:1985PNAS…82..708D. doi:10.1073/pnas.82.3.708. PMC 397115. PMID 2579379.
- “Entrez Gene: C3 complement component 3”.
- Sahu A, Lambris JD (Apr 2001). “Structure and biology of complement protein C3, a connecting link between innate and acquired immunity”. Immunological Reviews. 180: 35–48. doi:10.1034/j.1600-065X.2001.1800103.x. PMID 11414361. S2CID 21966958.
- Lachmann P (Dec 1975). “Genetics of the complement system”. Journal of Medical Genetics. 12 (4): 372–7. doi:10.1136/jmg.12.4.372. PMC 1013316. PMID 768477.
- Matsuyama W, Nakagawa M, Takashima H, Muranaga F, Sano Y, Osame M (Dec 2001). “Molecular analysis of hereditary deficiency of the third component of complement (C3) in two sisters”. Internal Medicine. 40 (12): 1254–8. doi:10.2169/internalmedicine.40.1254. PMID 11813855.
- Pasch, Marcel C.; van den Bosch, Norbert H.A.; Bos, Jan D.; Asghar, Syed S.; Daha, Mohamed R. (January 2000). “Synthesis of Complement Components C3 and Factor B in Human Keratinocytes is Differentially Regulated by Cytokines”. Journal of Investigative Dermatology. 114 (1): 78–82. doi:10.1046/j.1523-1747.2000.00841.x. PMID 10620119. Retrieved 28 August 2017.
- Ruseva, M M; Takahashi, M; Fujita, T; Pickering, M C (April 2014). “C3 dysregulation due to factor H deficiency is mannan-binding lectin-associated serine proteases (MASP)-1 and MASP-3 independent in vivo”. Clinical and Experimental Immunology. 176 (1): 84–92. doi:10.1111/cei.12244. ISSN 0009-9104. PMC 3958157. PMID 24279761.
Deficiency of C3 results in the affected person being immunocompromised. Specifically, they are vulnerable to bacterial pathogens, including repeat infections by the same organism, but are not susceptible to viruses. This vulnerability also occurs in an individual deficient in C1, C2, C4, or any of their required components or associated proteins, and the clinical effects are very similar regardless of the specific deficiency. This is because all of these must work with C3 for the complement system to function. Affected people are particularly vulnerable to infections with Gram-negative organisms such as pathogenic E. coli or Salmonella enterica. Additionally, C3 and other complement deficiencies are associated with frequent and severe respiratory infections, as well as other infections that invade and penetrate tissue layers. Some data shows that acquired C3 deficiency, including when this is intentionally done for medical immunosuppression purposes, may not significantly impact a person’s immune function long-term. However, by contrast, congenital C3 deficiency is known to cause chronic illness. Additionally, several forms of C3 deficiency contribute to the development of systemic lupus erythematosus and other autoimmune diseases.
- Fischer, Alain (2022). “Chapter 351: Primary Immune Deficiency Diseases”. Harrison’s Principles of Internal Medicine (21st ed.). New York: McGraw Hill. ISBN 978-1-264-26850-4.
- Lappegård, Knut Tore; Christiansen, Dorte; Pharo, Anne; Thorgersen, Ebbe Billmann; Hellerud, Bernt Christian; Lindstad, Julie; Nielsen, Erik Waage; Bergseth, Grethe; Fadnes, Dag; Abrahamsen, Tore G.; Høiby, E. Arne; Schejbel, Lone; Garred, Peter; Lambris, John D.; Harboe, Morten (2009-09-15). “Human genetic deficiencies reveal the roles of complement in the inflammatory network: Lessons from nature”. Proceedings of the National Academy of Sciences of the United States of America. 106 (37): 15861–15866. doi:10.1073/pnas.0903613106. ISSN 0027-8424. PMC 2732707. PMID 19717455.
- Reis, Edimara S.; Berger, Nadja; Wang, Xin; Koutsogiannaki, Sophia; Doot, Robert K.; Gumas, Justin T.; Foukas, Periklis G.; Resuello, Ranillo R.G.; Tuplano, Joel V.; Kukis, David; Tarantal, Alice F.; Young, Anthony J.; Kajikawa, Tetsuhiro; Soulika, Athena M.; Mastellos, Dimitrios C. (December 2018). “Safety profile after prolonged C3 inhibition”. Clinical Immunology. 197: 96–106. doi:10.1016/j.clim.2018.09.004. ISSN 1521-6616. PMC 6258316.


Leave a Reply