Human serum albumin
Human serum albumin is the serum albumin found in human blood. It is the most abundant protein in human blood plasma; it constitutes about half of serum protein. It is produced in the liver. It is soluble in water, and it is monomeric.[citation needed]
Albumin transports hormones, fatty acids, and other compounds, buffers pH, and maintains oncotic pressure, among other functions.
Albumin is synthesized in the liver as preproalbumin, which has an N-terminal peptide that is removed before the nascent protein is released from the rough endoplasmic reticulum. The product, proalbumin, is in turn cleaved in the Golgi apparatus to produce the secreted albumin.
The reference range for albumin concentrations in serum is approximately 35–50 g/L (3.5–5.0 g/dL). It has a serum half-life of approximately 21 days. It has a molecular mass of 66.5 kDa.
- “Harmonisation of Reference Intervals” (PDF). pathologyharmony.co.uk. Pathology Harmony. Archived from the original (PDF) on 2 August 2013. Retrieved 23 June 2013.
- “Hypoalbuminemia: Background, Pathophysiology, Etiology”. Medscape Reference. 2019-11-10. Retrieved 2019-12-22.
The gene for albumin is located on chromosome 4 in locus 4q13.3 and mutations in this gene can result in anomalous proteins. The human albumin gene is 16,961 nucleotides long from the putative ‘cap’ site to the first poly(A) addition site. It is split into 15 exons that are symmetrically placed within the 3 domains thought to have arisen by triplication of a single primordial domain.
Human serum albumin (HSA) is a highly water-soluble globular monomeric plasma protein with a relative molecular weight of 67 KDa, consisting of 585 amino acid residues, one sulfhydryl group and 17 disulfide bridges. Among nanoparticulate carriers, HSA nanoparticles have long been the center of attention in the pharmaceutical industry due to their ability to bind to various drug molecules, great stability during storage and in vivo usage, no toxicity and antigenicity, biodegradability, reproducibility, scale up of the production process and a better control over release properties. In addition, significant amounts of drug can be incorporated into the particle matrix because of the large number of drug binding sites on the albumin molecule.
- Kouchakzadeh H, Shojaosadati SA, Shokri F (September 2014). “Efficient loading and entrapment of tamoxifen in human serum albumin based nanoparticulate delivery system by a modified desolvation technique”. Chemical Engineering Research and Design. 92 (9): 1681–1692. doi:10.1016/j.cherd.2013.11.024.
Function
- Maintains oncotic pressure
- Transports thyroid hormones
- Transports other hormones, in particular, ones that are fat-soluble
- Transports fatty acids (“free” fatty acids) to the liver and to myocytes for utilization of energy
- Transports unconjugated bilirubin
- Transports many drugs; serum albumin levels can affect the half-life of drugs. Competition between drugs for albumin binding sites may cause drug interaction by increasing the free fraction of one of the drugs, thereby affecting potency.
- Competitively binds calcium ions (Ca2+)
- Serum albumin, as a negative acute-phase protein, is down-regulated in inflammatory states. As such, it is not a valid marker of nutritional status; rather, it is a marker of an inflammatory state
- Prevents photodegradation of folic acid
- Prevent pathogenic effects of Clostridioides difficile toxins
- di Masi A, Leboffe L, Polticelli F, Tonon F, Zennaro C, Caterino M, et al. (September 2018). “Human Serum Albumin Is an Essential Component of the Host Defense Mechanism Against Clostridium difficile Intoxication”. The Journal of Infectious Diseases. 218 (9): 1424–1435. doi:10.1093/infdis/jiy338. PMID 29868851.
Measurement
Serum albumin is commonly measured by recording the change in absorbance upon binding to a dye such as bromocresol green or bromocresol purple.
- “Albumin: analyte monograph” (PDF). Association for Clinical Biochemistry and Laboratory Medicine. Archived from the original (PDF) on 13 November 2012. Retrieved 23 June 2013.

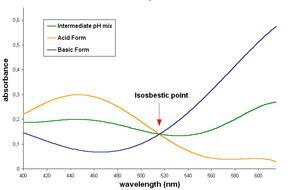
Bromocresol green (BCG) is a dye of the triphenylmethane family (triarylmethane dyes). It belongs to a class of dyes called sulfonephthaleins. It is used as a pH indicator in applications such as growth mediums for microorganisms and titrations. In clinical practise, it is commonly used as a diagnostic technique. The most common use of bromocresol green is to measure serum albumin concentration within mammalian blood samples in possible cases of kidney failure and liver disease. In chemistry, bromocresol green is used in Thin-layer chromatography staining solutions to visualize acidic compounds. In aqueous solution, bromocresol green will ionize to give the monoanionic form (yellow), that further deprotonates at higher pH to give the dianionic form (blue), which is stabilized by resonance. The acid dissociation constant (pKa) of this reaction is 4.8. Tap water is sufficiently basic to give a solution of bromocresol green its characteristic blue-green color. The acid and basic forms of this dye have an isosbestic point in their UV-Visible spectrum, around 515 nm, indicate that the two forms interconvert directly without forming any other substance. An ethanol solution (0.04 wt%) of bromocresol green has been proposed for TLC staining and is suitable for visualisation of the compounds with functional groups whose pKa is below 5.0 (carboxylic acids, sulfonic acids, etc.). These appear as yellow spots on a light or dark blue background; no heating is necessary. Bromophenol blue solution can be used for the same purpose. The compound is synthesized by bromination of cresol purple (m-cresolsulfonphthalein).
Uses
It is used as a pH indicator and as a tracking dye for DNA agarose gel electrophoresis. It can be used in its free acid form (light brown solid), or as a sodium salt (dark green solid). It is also an inhibitor of the prostaglandin E2 transport protein. ??? Additional applications include use in sol-gel matrices, the detection of ammonia, and the measurement of albumin in human plasma and serum. Bromocresol green may cause irritation. Skin and eye contact should be avoided.
Bromocresol purple (BCP) or 5′,5″-dibromo-o-cresolsulfophthalein, is a dye of the triphenylmethane family (triarylmethane dyes) and a pH indicator. It is colored yellow below pH 5.2, and violet above pH 6.8. In its cyclic sulfonate ester form, it has a pKa value of 6.3, and is usually prepared as a 0.04% aqueous solution. Bromocresol purple is used in medical laboratories to measure albumin. Use of BCP in this application may provide some advantage over older methods using bromocresol green. In microbiology, it is used for staining dead cells based on their acidity, and for the isolation and assaying of lactic acid bacteria. In photographic processing, it can be used as an additive to acid stop baths to indicate that the bath has reached neutral pH and needs to be replaced. Bromocresol purple milk solids glucose agar is used as a medium used to distinguish dermatophytes from bacteria and other organisms in cases of ringworm fungus (T. verrucosum) infestation in cattle and other animals.
pH Indicator
Similar to bromocresol green, the structure of bromocresol purple changes with pH. Changing the level of acidity causes a shift in the equilibrium between two different structures that have different colors. In near-neutral or alkaline solution, the chemical has a sulfonate structure that gives the solution a purple color. As the pH decreases, it converts to a sultone (cyclic sulfonic ester) that colors the solution yellow. In some microbiology tests, this change is used as an indicator of bacterial growth.
- “Bromocresol green”.
- Kolthoff, I.M. Treatise on Analytical Chemistry, New York, Interscience Encyclopedia, Inc., 1959.
- “Bromocresol Green”. Sigma Aldrich.
- Sabnis, R. W. (2008). Handbook of Acid-Base Indicators. Boca Raton, FL: CRC Press. pp. 43–44. ISBN 9780849382185.
- Fred Senese. “Acid-Base Indicators”. Frostburg State University Dept. of Chemistry.
- Diamond, D.; Lau, K. T.; Brady, S.; Cleary, J. (2008). “Integration of analytical measurements and wireless communications—Current issues and future strategies”. Talanta. 75 (3): 606–12. doi:10.1016/j.talanta.2007.11.022. PMID 18585121.
- Anonymous. Bromocresol Green. In The Merck index : an encyclopedia of chemicals, drugs, and biologicals; Windholz, M., Ed.; Merck & Co., Inc.: Rahway, N.J., 1983; pp 191.
- “Bromocresol Purple”. NCBI PubChem. National Center for Biotechnology Information.
- Bachmann, Lorin M.; Yu, Min; Boyd, James C.; Bruns, David E.; Miller, W. Greg (2017-03-01). “State of Harmonization of 24 Serum Albumin Measurement Procedures and Implications for Medical Decisions”. Clinical Chemistry. 63 (3): 770–779. doi:10.1373/clinchem.2016.262899. ISSN 0009-9147. PMID 28073902.
- Ito, Shigenori; Yamamoto, Daisuke (2010-02-02). “Mechanism for the color change in bromocresol purple bound to human serum albumin”. Clinica Chimica Acta. 411 (3): 294–295. doi:10.1016/j.cca.2009.11.019. PMID 19932090.
- Kurzweilová, H.; Sigler, K. (November 1993). “Fluorescent staining with bromocresol purple: a rapid method for determining yeast cell dead count developed as an assay of killer toxin activity”. Yeast. 9 (11): 1207–1211. doi:10.1002/yea.320091107. PMID 7509098. S2CID 44782970.
- Lee, H.M.; Lee, Y. (June 2008). “A differential medium for lactic acid-producing bacteria in a mixed culture”. Letters in Applied Microbiology. 46 (6): 676–681. doi:10.1111/j.1472-765X.2008.02371.x. PMID 18444977.
- Anchell, Steve (2016). The Darkroom Cookbook (4 ed.). Routledge. ISBN 9781317337607 – via Google Books.
- Kane, J.; Summerbell, R.; Sigler, L.; Krajden, S.; Land, G. (1997). Laboratory Handbook of Dermatophytes: A Clinical Guide and Laboratory Handbook of Dermatophytes and Other Filamentous Fungi from Skin, Hair, and Nails. Belmont, CA: Star Publishing Company. ISBN 9780898631579.
- Beneke, E. S.; Rogers, A. L. (1996). Medical Mycology and Human Mycoses (illustrated ed.). Belmont, CA: Star Publishing Company. pp. 85–90. ISBN 9780898631753.
- “Bromocresol Purple – an overview | ScienceDirect Topics”.
- “Archived copy”. Archived from the original on 2022-02-16. Retrieved 2022-02-15.
Reference ranges
The normal range of human serum albumin in adults (> 3 y.o.) is 3.5–5.0 g/dL (35–50 g/L). For children less than three years of age, the normal range is broader, 2.9–5.5 g/dL.
- “Normal Ranges for Common Laboratory Tests”. Archived from the original on 2013-01-14. Retrieved 2007-12-06. Rush University ???
Low albumin (hypoalbuminemia) may be caused by liver disease, nephrotic syndrome, burns, protein-losing enteropathy, malabsorption, malnutrition, late pregnancy, artefact, genetic variations and malignancy.[citation needed]
High albumin (hyperalbuminemia) is almost always caused by dehydration. In some cases of retinol (Vitamin A) deficiency, the albumin level can be elevated to high-normal values (e.g., 4.9 g/dL) because retinol causes cells to swell with water. (This is also the reason too much Vitamin A is toxic.) This swelling also likely occurs during treatment with 13-cis retinoic acid (isotretinoin), a pharmaceutical for treating severe acne, amongst other conditions. In lab experiments it has been shown that all-trans retinoic acid down regulates human albumin production.
- Pasantes-Morales H, Wright CE, Gaull GE (December 1984). “Protective effect of taurine, zinc and tocopherol on retinol-induced damage in human lymphoblastoid cells”. The Journal of Nutrition. 114 (12): 2256–2261. doi:10.1093/jn/114.12.2256. PMID 6502269.
- Masaki T, Matsuura T, Ohkawa K, Miyamura T, Okazaki I, Watanabe T, Suzuki T (July 2006). “All-trans retinoic acid down-regulates human albumin gene expression through the induction of C/EBPbeta-LIP”. The Biochemical Journal. 397 (2): 345–353. doi:10.1042/BJ20051863. PMC 1513275. PMID 16608438.
Isotretinoin, also known as 13-cis-retinoic acid and sold under the brand name Accutane among others, is a medication primarily used to treat severe acne. It is also used to prevent certain skin cancers (squamous-cell carcinoma), and in the treatment of other cancers. It is used to treat harlequin-type ichthyosis, a usually lethal skin disease, and lamellar ichthyosis. It is a retinoid, meaning it is related to vitamin A, and is found in small quantities naturally in the body. Its isomer, tretinoin, is also an acne drug. The most common adverse effects are dry lips (cheilitis), dry and fragile skin, and an increased susceptibility to sunburn. Uncommon and rare side effects include muscle aches and pains (myalgias), and headaches. Isotretinoin is known to cause birth defects due to in-utero exposure because of the molecule’s close resemblance to retinoic acid, a natural vitamin A derivative that controls normal embryonic development. It is also associated with psychiatric side effects, most commonly depression but also, more rarely, psychosis and unusual behaviours. Other rare side effects include hyperostosis and premature epiphyseal closure, which have been reported to be persistent. Isotretinoin was patented in 1969 and approved for medical use in 1982. In 2021, it was the 264th most commonly prescribed medication in the United States, with more than 1 million prescriptions. In February 2002, Roche’s patents for isotretinoin expired, and there are now many other companies selling cheaper generic versions of the drug. On 29 June 2009, Roche Pharmaceuticals, the original creator and distributor of isotretinoin, officially discontinued both the manufacture and distribution of their Accutane brand in the United States due to what the company described as business reasons related to low market share (below 5%), coupled with the high cost of defending personal injury lawsuits brought by some people who took the drug. Roche USA continues to defend Accutane and claims to have treated over 13 million people since its introduction in 1982. F. Hoffmann-La Roche Ltd. apparently will continue to manufacture and distribute Roaccutane outside of the United States. Among others, actor James Marshall sued Roche over allegedly Accutane-related disease that resulted in removal of his colon. The jury, however, decided that James Marshall had a pre-existing bowel disease. Several trials over inflammatory bowel disease claims have been held in the United States, with many of them resulting in multimillion-dollar judgments against the makers of isotretinoin. As of 2017, it was marketed as a topical combination drug with erythromycin under the brand names Isotrex Eritromicina, Isotrexin, and Munderm. As of 2017, isotretinoin was marketed under many brand names worldwide: A-Cnotren, Absorica, Accuran, Accutane, Accutin, Acne Free, Acnecutan, Acnegen, Acnemin, Acneone, Acneral, Acnestar, Acnetane, Acnetin A, Acnetrait, Acnetrex, Acnogen, Acnotin, Acnotren, Acretin, Actaven, Acugen, Acutret, Acutrex, Ai Si Jie, Aisoskin, Aknal, Aknefug Iso, Aknenormin, Aknesil, Aknetrent, Amnesteem, Atlacne, Atretin, Axotret, Casius, Ciscutan, Claravis, Contracné, Curacne, Curacné, Curakne, Curatane, Cuticilin, Decutan, Dercutane, Effederm, Epuris, Eudyna, Farmacne, Flexresan, Flitrion, I-Ret, Inerta, Inflader, Inotrin, Isac, Isdiben, Isoacne, Isobest, Isocural, Isoderm, Isoface, IsoGalen, Isogeril, Isolve, Isoprotil, Isoriac, Isosupra, Isosupra Lidose, Isotane, Isotina, Isotinon, Isotren, Isotret, Isotretinoin, Isotretinoina, Isotretinoína, Isotretinoine, Isotretinoïne, Isotrétinoïne, Isotretinoinum, Isotrex, Isotrin, Isotroin, Izotek, Izotziaja, Lisacne, Locatret, Mayesta, Medinac, Myorisan, Neotrex, Netlook, Nimegen, Noitron, Noroseptan, Novacne, Oralne, Oraret, Oratane, Piplex, Policano, Procuta, Reducar, Retacnyl, Retin A, Roaccutan, Roaccutane, Roacnetan, Roacta, Roacutan, Rocne, Rocta, Sotret, Stiefotrex, Tai Er Si, Teweisi, Tretin, Tretinac, Tretinex, Tretiva, Tufacne, Zenatane, Zerocutan, Zonatian ME, and Zoretanin.
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 476. ISBN 978-3-527-60749-5.
- “The Top 300 of 2021”. ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- “Isotretinoin – Drug Usage Statistics”. ClinCalc. Retrieved 14 January 2024.
- “Isotretinoin international brands”. Drugs.com. Retrieved 1 June 2017.
- Wysowski DK, Swartz L (May 2005). “Relationship between headache and depression in users of isotretinoin”. Archives of Dermatology. 141 (5): 640–1. doi:10.1001/archderm.141.5.640. PMID 15897395.
- Magin P, Pond D, Smith W (February 2005). “Isotretinoin, depression and suicide: a review of the evidence”. The British Journal of General Practice. 55 (511): 134–8. PMC 1463189. PMID 15720936.
- Ng CH, Schweitzer I (February 2003). “The association between depression and isotretinoin use in acne”. The Australian and New Zealand Journal of Psychiatry. 37 (1): 78–84. doi:10.1046/j.1440-1614.2003.01111.x. PMID 12534661. S2CID 8475675.
- “FDA information, side effects, and uses / Accutane (isotretinoin)”. U. S. Food and Drug Administration (FDA). Retrieved 20 January 2014.
- “FDA information, side effects, and uses / Accutane (isotretinoin) : Table 2 Pharmacokinetic Parameters of Isotretinoin Mean (%CV), N=74“. U. S. Food and Drug Administration (FDA). Retrieved 20 January 2014.
- “FDA information, side effects, and uses / Accutane (isotretinoin) : Drug Interactions”. U. S. Food and Drug Administration (FDA). Retrieved 20 January 2014.
- Abramowitz M, Hilts P (23 April 1988). “FDA Eyes Ban on Acne Drug”. Washington Post. Retrieved 14 November 2022.
- “Anti-Acne Drug Faulted in Birth Defects”. The New York Times. 22 April 1988. Retrieved 2 December 2022.
- Centers for Disease Control Prevention (CDC) (January 2000). “Accutane-exposed pregnancies–California, 1999” (PDF). MMWR. Morbidity and Mortality Weekly Report. 49 (2): 28–31. PMID 10680601.
- Choi JS, Koren G, Nulman I (March 2013). “Pregnancy and isotretinoin therapy”. CMAJ. 185 (5): 411–413. doi:10.1503/cmaj.120729. PMC 3602257. PMID 23296582.
- Roan S (7 November 2009). “New study may deal final blow to acne drug Accutane”. Los Angeles Times.
- “Roche Discontinues and Plans to Delist Accutane in the U.S.” (Press release). Genentech. 29 June 2009. Archived from the original on 8 November 2009. Retrieved 12 November 2010.
- Feeley J (11 March 2011). “Roche Accutane Acne Drug Caused ‘Tragedy’ for Actor, Brian Dennehy Says”. Bloomberg.
- Silverman E (4 November 2011). “It’s Curtains On Actor’s Accutane Lawsuit”. Pharmalot. UBM Canon.[permanent dead link]
- Voreacos D (30 May 2007). “Roche Found Liable in First Of 400 Suits Over Accutane”. The Washington Post. Bloomberg News. Retrieved 30 April 2012.
Pathology
Hypoalbuminemia
Hypoalbuminemia means low blood albumin levels.
- Anderson DM (2000). Dorland’s illustrated medical dictionary (29th ed.). Philadelphia [u.a.]: Saunders. p. 860. ISBN 978-0721682617.
This can be caused by:
- Liver disease; cirrhosis of the liver is most common
- Excess excretion by the kidneys (as in nephrotic syndrome)
- Excess loss in bowel (protein-losing enteropathy, e.g., Ménétrier’s disease)
- Burns (plasma loss in the absence of skin barrier)
- Redistribution (hemodilution [as in pregnancy], increased vascular permeability or decreased lymphatic clearance)
- Acute disease states (referred to as a negative acute-phase protein)
- Zerbato V, Sanson G, De Luca M, Di Bella S, di Masi A, Caironi P, et al. (2022-04-20). “The Impact of Serum Albumin Levels on COVID-19 Mortality”. Infectious Disease Reports. 14 (3): 278–286. doi:10.3390/idr14030034. ISSN 2036-7449. PMC 9149867. PMID 35645213.
- Malnutrition and wasting
- Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, et al. (September 2012). “The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience”. JACC. Cardiovascular Interventions. 5 (9): 974–981. doi:10.1016/j.jcin.2012.06.011. PMC 3717525. PMID 22995885.
- Mutation causing analbuminemia (very rare)
- Anorexia nervosa (most common cause in adolescents)
In clinical medicine, hypoalbuminemia significantly correlates with a higher mortality rates in several conditions such as heart failure, post-surgery, COVID-19.
- Uthamalingam S, Kandala J, Daley M, Patvardhan E, Capodilupo R, Moore SA, Januzzi JL (December 2010). “Serum albumin and mortality in acutely decompensated heart failure”. American Heart Journal. 160 (6): 1149–1155. doi:10.1016/j.ahj.2010.09.004. PMID 21146671.
- Xu R, Hao M, Zhou W, Liu M, Wei Y, Xu J, Zhang W (August 2022). “Preoperative hypoalbuminemia in patients undergoing cardiac surgery: a meta-analysis”. Surgery Today. 53 (8): 861–872. doi:10.1007/s00595-022-02566-9. PMID 35933630. S2CID 251369303.
- Zerbato V, Sanson G, De Luca M, Di Bella S, di Masi A, Caironi P, et al. (April 2022). “The Impact of Serum Albumin Levels on COVID-19 Mortality”. Infectious Disease Reports. 14 (3): 278–286. doi:10.3390/idr14030034. PMC 9149867. PMID 35645213.
Hyperalbuminemia
Hyperalbuminemia is an increased concentration of albumin in the blood. Typically, this condition is due to dehydration. Hyperalbuminemia has also been associated with high protein diets.
- Busher JT (1990). “Chapter 101: Serum Albumin and Globulin”. In Walker HK, Hall WD, Hurst JW (eds.). Clinical methods : the history, physical, and laboratory examinations (3rd ed.). Boston: Butterworths. ISBN 978-0409900774. PMID 21250048.
- Mutlu EA, Keshavarzian A, Mutlu GM (June 2006). “Hyperalbuminemia and elevated transaminases associated with high-protein diet”. Scandinavian Journal of Gastroenterology. 41 (6): 759–760. doi:10.1080/00365520500442625. PMID 16716979. S2CID 21264934.
Medical use
Human albumin solution (HSA) is available for medical use, usually at concentrations of 5–25%.
Human albumin is often used to replace lost fluid and help restore blood volume in trauma, burns and surgery patients. There is no strong medical evidence that albumin administration (compared to saline) saves lives for people who have hypovolaemia or for those who are critically ill due to burns or hypoalbuminaemia. It is also not known if there are people who are critically ill that may benefit from albumin. Therefore, the Cochrane Collaboration recommends that it should not be used, except in clinical trials.
- Roberts I, Blackhall K, Alderson P, Bunn F, Schierhout G (November 2011). “Human albumin solution for resuscitation and volume expansion in critically ill patients”. The Cochrane Database of Systematic Reviews. 2011 (11): CD001208. doi:10.1002/14651858.CD001208.pub4. hdl:2299/5243. PMC 7055200. PMID 22071799.
- Yu YT, Liu J, Hu B, Wang RL, Yang XH, Shang XL, et al. (July 2021). “Expert consensus on the use of human serum albumin in critically ill patients“. Chinese Medical Journal. 134 (14): 1639–1654. doi:10.1097/CM9.0000000000001661. PMC 8318641. PMID 34397592.
In acoustic droplet vaporization (ADV), albumin is sometimes used as a surfactant. ADV has been proposed as a cancer treatment by means of occlusion therapy.
- Lo AH, Kripfgans OD, Carson PL, Rothman ED, Fowlkes JB (May 2007). “Acoustic droplet vaporization threshold: effects of pulse duration and contrast agent”. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 54 (5): 933–946. doi:10.1109/tuffc.2007.339. PMID 17523558. S2CID 11983041.
Acoustic droplet vaporization (ADV) is the process by which superheated liquid droplets are phase-transitioned into gas bubbles by means of ultrasound. Perfluorocarbons and halocarbons are often used for the dispersed medium, which forms the core of the droplet. The surfactant, which forms a stabilizing shell around the dispersive medium, is usually composed of albumin or lipids. There exist two main hypothesis that explain the mechanism by which ultrasound induces vaporization. One poses that the ultrasonic field interacts with the dispersed medium so as to cause vaporization in the bubble core. The other suggests that shockwaves from inertial cavitation, occurring near or within the droplet, cause the dispersed medium to vaporize.
- Carson, Paul L.; et al. “Acoustic Droplet Vaporization”. University of Michigan Basic Radiological Sciences and Ultrasound Group. University of Michigan. Archived from the original on 16 January 2013. Retrieved 30 June 2013.
- Kripfgans, Oliver D. (July 2004). “On the acoustic vaporization of micrometer-sized droplets”. Journal of the Acoustical Society of America. 116 (1): 272–281. Bibcode:2004ASAJ..116..272K. doi:10.1121/1.1755236. PMID 15295987.
See also
Human serum albumin may be used to potentially reverse drug/chemical toxicity by binding to free drug/agent.
- Ascenzi P, Leboffe L, Toti D, Polticelli F, Trezza V (August 2018). “Fipronil recognition by the FA1 site of human serum albumin”. Journal of Molecular Recognition. 31 (8): e2713. doi:10.1002/jmr.2713. PMID 29656610. S2CID 4894574.
Human albumin may also be used in treatment of decompensated cirrhosis.
- Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, et al. (June 2018). “Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial”. Lancet. 391 (10138): 2417–2429. doi:10.1016/S0140-6736(18)30840-7. hdl:2108/208667. PMID 29861076. S2CID 44120418.
Human serum albumin has been used as a component of a frailty index.
- Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, et al. (September 2012). “The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience”. JACC. Cardiovascular Interventions. 5 (9): 974–981. doi:10.1016/j.jcin.2012.06.011. PMC 3717525. PMID 22995885.
Glycation
It has been known for a long time that human blood proteins like hemoglobin and serum albumin may undergo a slow non-enzymatic glycation, mainly by formation of a Schiff base between ε-amino groups of lysine (and sometimes arginine) residues and glucose molecules in blood (Maillard reaction). This reaction can be inhibited in the presence of antioxidant agents. Although this reaction may happen normally, elevated glycoalbumin is observed in diabetes mellitus.
- Rahbar S (October 1968). “An abnormal hemoglobin in red cells of diabetics”. Clinica Chimica Acta; International Journal of Clinical Chemistry. 22 (2): 296–298. doi:10.1016/0009-8981(68)90372-0. PMID 5687098.
- Day JF, Thorpe SR, Baynes JW (February 1979). “Nonenzymatically glucosylated albumin. In vitro preparation and isolation from normal human serum”. The Journal of Biological Chemistry. 254 (3): 595–597. doi:10.1016/S0021-9258(17)37845-6. PMID 762083.
- Iberg N, Flückiger R (October 1986). “Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites”. The Journal of Biological Chemistry. 261 (29): 13542–13545. doi:10.1016/S0021-9258(18)67052-8. PMID 3759977.
- Jakus V, Hrnciarová M, Cársky J, Krahulec B, Rietbrock N (1999). “Inhibition of nonenzymatic protein glycation and lipid peroxidation by drugs with antioxidant activity”. Life Sciences. 65 (18–19): 1991–1993. doi:10.1016/S0024-3205(99)00462-2. PMID 10576452.
Glycation has the potential to alter the biological structure and function of the serum albumin protein.
- Mohamadi-Nejad A, Moosavi-Movahedi AA, Hakimelahi GH, Sheibani N (September 2002). “Thermodynamic analysis of human serum albumin interactions with glucose: insights into the diabetic range of glucose concentration”. The International Journal of Biochemistry & Cell Biology. 34 (9): 1115–1124. doi:10.1016/S1357-2725(02)00031-6. PMID 12009306.
- Shaklai N, Garlick RL, Bunn HF (March 1984). “Nonenzymatic glycosylation of human serum albumin alters its conformation and function”. The Journal of Biological Chemistry. 259 (6): 3812–3817. doi:10.1016/S0021-9258(17)43168-1. PMID 6706980.
- Mendez DL, Jensen RA, McElroy LA, Pena JM, Esquerra RM (December 2005). “The effect of non-enzymatic glycation on the unfolding of human serum albumin”. Archives of Biochemistry and Biophysics. 444 (2): 92–99. doi:10.1016/j.abb.2005.10.019. PMID 16309624.
- Mohamadi-Nejada A, Moosavi-Movahedi AA, Safariana S, Naderi-Maneshc MH, Ranjbarc B, Farzamid B, Mostafavie H, Larijanif MB, Hakimelahi GH (July 2002). “The thermal analysis of nonezymatic glycosylation of human serum albumin: differential scanning calorimetry and circular dichroism studies”. Thermochimica Acta. 389 (1–2): 141–151. doi:10.1016/S0040-6031(02)00006-0.
Moreover, the glycation can result in the formation of Advanced Glycation End-Products (AGE), which result in abnormal biological effects. Accumulation of AGEs leads to tissue damage via alteration of the structures and functions of tissue proteins, stimulation of cellular responses, through receptors specific for AGE-proteins, and generation of reactive oxygen intermediates. AGEs also react with DNA, thus causing mutations and DNA transposition. Thermal processing of proteins and carbohydrates brings major changes in allergenicity. AGEs are antigenic and represent many of the important neoantigens found in cooked or stored foods. They also interfere with the normal product of nitric oxide in cells.
- Kańska U, Boratyński J (2002). “Thermal glycation of proteins by D-glucose and D-fructose”. Archivum Immunologiae et Therapiae Experimentalis. 50 (1): 61–66. PMID 11916310.
- Rojas A, Romay S, González D, Herrera B, Delgado R, Otero K (February 2000). “Regulation of endothelial nitric oxide synthase expression by albumin-derived advanced glycosylation end products”. Circulation Research. 86 (3): E50–E54. doi:10.1161/01.RES.86.3.e50. PMID 10679490.
Although there are several lysine and arginine residues in the serum albumin structure, very few of them can take part in the glycation reaction.
- Iberg N, Flückiger R (October 1986). “Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites”. The Journal of Biological Chemistry. 261 (29): 13542–13545. doi:10.1016/S0021-9258(18)67052-8. PMID 3759977
- Garlick RL, Mazer JS (May 1983). “The principal site of nonenzymatic glycosylation of human serum albumin in vivo”. The Journal of Biological Chemistry. 258 (10): 6142–6146. doi:10.1016/S0021-9258(18)32384-6. PMID 6853480.
Oxidation
The albumin is the predominant protein in most body fluids, its Cys34 represents the largest fraction of free thiols within the body. The albumin Cys34 thiol exists in both reduced and oxidized forms. In plasma of healthy young adults, 70–80% of total HSA contains the free sulfhydryl group of Cys34 in a reduced form or mercaptoalbumin (HSA-SH). However, in pathological states characterized by oxidative stress such as kidney disease, liver disease and diabetes the oxidized form, or non-mercaptoalbumin (HNA), could predominate. The albumin thiol reacts with radical hydroxyl (.OH), hydrogen peroxide (H2O2) and the reactive nitrogen species as peroxynitrite (ONOO.), and have been shown to oxidize Cys34 to sulfenic acid derivate (HSA-SOH), it can be recycled to mercapto-albumin; however at high concentrations of reactive species leads to the irreversible oxidation to sulfinic (HSA-SO2H) or sulfonic acid (HSA-SO3H) affecting its structure. Presence of reactive oxygen species (ROS), can induce irreversible structural damage and alter protein activities.[citation needed]
- Kawakami A, Kubota K, Yamada N, Tagami U, Takehana K, Sonaka I, et al. (July 2006). “Identification and characterization of oxidized human serum albumin. A slight structural change impairs its ligand-binding and antioxidant functions”. The FEBS Journal. 273 (14): 3346–3357. doi:10.1111/j.1742-4658.2006.05341.x. PMID 16857017. S2CID 12844381.
- Turell L, Carballal S, Botti H, Radi R, Alvarez B (April 2009). “Oxidation of the albumin thiol to sulfenic acid and its implications in the intravascular compartment”. Brazilian Journal of Medical and Biological Research = Revista Brasileira de Pesquisas Medicas e Biologicas. 42 (4): 305–311. doi:10.1590/s0100-879×2009000400001. PMID 19330257.
- Rosas-Díaz M, Camarillo-Cadena M, Hernández-Arana A, Ramón-Gallegos E, Medina-Navarro R (June 2015). “Antioxidant capacity and structural changes of human serum albumin from patients in advanced stages of diabetic nephropathy and the effect of the dialysis”. Molecular and Cellular Biochemistry. 404 (1–2): 193–201. doi:10.1007/s11010-015-2378-2. PMID 25758354. S2CID 6718332.
- Watanabe H, Imafuku T, Otagiri M, Maruyama T (2017). “Clinical Implications Associated With the Posttranslational Modification-Induced Functional Impairment of Albumin in Oxidative”. Journal of Pharmaceutical Sciences. 106 (9): 2195–2203. doi:10.1016/j.xphs.2017.03.002. PMID 28302542.
- Matsuyama Y, Terawaki H, Terada T, Era S (August 2009). “Albumin thiol oxidation and serum protein carbonyl formation are progressively enhanced with advancing stages of chronic kidney disease”. Clinical and Experimental Nephrology. 13 (4): 308–315. doi:10.1007/s10157-009-0161-y. PMID 19363646. S2CID 20886185.
Loss via kidneys
In the healthy kidney, albumin’s size and negative electric charge exclude it from excretion in the glomerulus. This is not always the case, as in some diseases including diabetic nephropathy, which can sometimes be a complication of uncontrolled or of longer term diabetes in which proteins can cross the glomerulus. The lost albumin can be detected by a simple urine test. Depending on the amount of albumin lost, a patient may have normal renal function, microalbuminuria, or albuminuria.
- “Microalbumin Urine Test”. WebMD.
Interactions
Human serum albumin has been shown to interact with FCGRT.
- Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL (February 2003). “The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan”. The Journal of Experimental Medicine. 197 (3): 315–322. doi:10.1084/jem.20021829. PMC 2193842. PMID 12566415.
The neonatal fragment crystallizable (Fc) receptor (also FcRn, IgG receptor FcRn large subunit p51, or Brambell receptor) is a protein that in humans is encoded by the FCGRTgene. It is an IgG Fc receptor which is similar in structure to the MHC class I molecule and also associates with beta-2-microglobulin. In rodents, FcRn was originally identified as the receptor that transports maternal immunoglobulin G (IgG) from mother to neonatal offspring via mother’s milk, leading to its name as the neonatal Fc receptor. In humans, FcRn is present in the placenta where it transports mother’s IgG to the growing fetus. FcRn has also been shown to play a role in regulating IgG and serum albumin turnover. Neonatal Fc receptor expression is up-regulated by the proinflammatory cytokine, TNF, and down-regulated by IFN-γ.
- Story CM, Mikulska JE, Simister NE (December 1994). “A major histocompatibility complex class I-like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus”. The Journal of Experimental Medicine. 180 (6): 2377–2381. doi:10.1084/jem.180.6.2377. PMC 2191771. PMID 7964511.
- Kandil E, Egashira M, Miyoshi O, Niikawa N, Ishibashi T, Kasahara M, Miyosi O (July 1996). “The human gene encoding the heavy chain of the major histocompatibility complex class I-like Fc receptor (FCGRT) maps to 19q13.3”. Cytogenetics and Cell Genetics. 73 (1–2): 97–98. doi:10.1159/000134316. PMID 8646894.
- “Entrez Gene: FCGRT Fc fragment of IgG, receptor, transporter, alpha”.
- Simister NE, Mostov KE (1989). “Cloning and expression of the neonatal rat intestinal Fc receptor, a major histocompatibility complex class I antigen homolog”. Cold Spring Harbor Symposia on Quantitative Biology. 54 (Pt 1): 571–580. doi:10.1101/sqb.1989.054.01.068. PMID 2534798.
- Kuo TT, Aveson VG (2011-01-01). “Neonatal Fc receptor and IgG-based therapeutics”. mAbs. 3 (5): 422–430. doi:10.4161/mabs.3.5.16983. PMC 3225846. PMID 22048693.
- Rodewald R, Kraehenbuhl JP (July 1984). “Receptor-mediated transport of IgG”. The Journal of Cell Biology. 99 (1 Pt 2): 159s–164s. doi:10.1083/jcb.99.1.159s. PMC 2275593. PMID 6235233.
- Simister NE, Rees AR (July 1985). “Isolation and characterization of an Fc receptor from neonatal rat small intestine”. European Journal of Immunology. 15 (7): 733–738. doi:10.1002/eji.1830150718. PMID 2988974. S2CID 42396197.
- Firan M, Bawdon R, Radu C, Ober RJ, Eaken D, Antohe F, et al. (August 2001). “The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans”. International Immunology. 13 (8): 993–1002. doi:10.1093/intimm/13.8.993. PMID 11470769.
- Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES (March 1996). “Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice”. European Journal of Immunology. 26 (3): 690–696. doi:10.1002/eji.1830260327. PMID 8605939. S2CID 85730132.
- Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL (February 2003). “The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan”. The Journal of Experimental Medicine. 197 (3): 315–322. doi:10.1084/jem.20021829. PMC 2193842. PMID 12566415.
- Ghetie V, Popov S, Borvak J, Radu C, Matesoi D, Medesan C, et al. (July 1997). “Increasing the serum persistence of an IgG fragment by random mutagenesis”. Nature Biotechnology. 15 (7): 637–640. doi:10.1038/nbt0797-637. PMID 9219265. S2CID 39836528.
- Roopenian DC, Akilesh S (September 2007). “FcRn: the neonatal Fc receptor comes of age”. Nature Reviews. Immunology. 7 (9): 715–725. doi:10.1038/nri2155. PMID 17703228. S2CID 6980400.
- Ward ES, Ober RJ (2009). Chapter 4: Multitasking by exploitation of intracellular transport functions the many faces of FcRn. Advances in Immunology. Vol. 103. pp. 77–115. doi:10.1016/S0065-2776(09)03004-1. ISBN 9780123748324. PMC 4485553. PMID 19755184.
- Kuo TT, Baker K, Yoshida M, Qiao SW, Aveson VG, Lencer WI, Blumberg RS (November 2010). “Neonatal Fc receptor: from immunity to therapeutics”. Journal of Clinical Immunology. 30 (6): 777–789. doi:10.1007/s10875-010-9468-4. PMC 2970823. PMID 20886282.
It might also interact with a yet-unidentified albondin (gp60), a certain pair of gp18/gp30, and some other proteins like:
- osteonectin (make separate note)
- Osteonectin (ON) also known as secreted protein acidic and rich in cysteine (SPARC) or basement-membrane protein 40 (BM-40) is a protein that in humans is encoded by the SPARC gene. Osteonectin is a glycoprotein in the bone that binds calcium. It is secreted by osteoblasts during bone formation, initiating mineralization and promoting mineral crystal formation. Osteonectin also shows affinity for collagen in addition to bone mineral calcium. A correlation between osteonectin over-expression and ampullary cancers and chronic pancreatitis has been found. Osteonectin is expressed by a wide variety of cells, including chondrocytes, fibroblasts, platelets, endothelial cells, epithelial cells, Leydig cells, Sertoli cells, luteal cells, adrenal cortical cells, and numerous neoplastic cell lines (such as SaOS-2 cells from human osteosarcoma). Osteonectin is an acidic extracellular matrix glycoprotein that plays a vital role in bone mineralization, cell-matrix interactions, and collagen binding. Osteonectin also increases the production and activity of matrix metalloproteinases, a function important to invading cancer cells within bone. Additional functions of osteonectin beneficial to tumor cells include angiogenesis, proliferation and migration. Overexpression of osteonectin is reported in many human cancers such as breast, prostate, colon and pancreatic. This molecule has been implicated in several biological functions, including mineralization of bone and cartilage, inhibiting mineralization, modulation of cell proliferation, facilitation of acquisition of differentiated phenotype and promotion of cell attachment and spreading. A number of phosphoproteins and glycoproteins are found in bone. The phosphate is bound to the protein backbone through phosphorylated serine or threonine amino acid residues. The best characterized of these bone proteins is osteonectin. It binds collagen and hydroxyapatite in separate domains, is found in relatively large amounts in immature bone, and promotes mineralization of collagen.
- Guweidhi A, Kleeff J, Adwan H, Giese NA, Wente MN, Giese T, Büchler MW, Berger MR, Friess H (Aug 2005). “Osteonectin influences growth and invasion of pancreatic cancer cells”. Annals of Surgery. 242 (2): 224–34. doi:10.1097/01.sla.0000171866.45848.68. PMC 1357728. PMID 16041213.
- Wasi S, Otsuka K, Yao KL, Tung PS, Aubin JE, Sodek J, Termine JD (Jun 1984). “An osteonectinlike protein in porcine periodontal ligament and its synthesis by periodontal ligament fibroblasts”. Canadian Journal of Biochemistry and Cell Biology. 62 (6): 470–8. doi:10.1139/o84-064. PMID 6380686.
- Young MF, Kerr JM, Ibaraki K, Heegaard AM, Robey PG (Aug 1992). “Structure, expression, and regulation of the major noncollagenous matrix proteins of bone”. Clinical Orthopaedics and Related Research. 281 (281): 275–94. doi:10.1097/00003086-199208000-00042. PMID 1499220.
- Lane TF, Sage EH (Feb 1994). “The biology of SPARC, a protein that modulates cell-matrix interactions”. FASEB Journal. 8 (2): 163–73. doi:10.1096/fasebj.8.2.8119487. PMID 8119487. S2CID 32958146.
- Osteonectin (ON) also known as secreted protein acidic and rich in cysteine (SPARC) or basement-membrane protein 40 (BM-40) is a protein that in humans is encoded by the SPARC gene. Osteonectin is a glycoprotein in the bone that binds calcium. It is secreted by osteoblasts during bone formation, initiating mineralization and promoting mineral crystal formation. Osteonectin also shows affinity for collagen in addition to bone mineral calcium. A correlation between osteonectin over-expression and ampullary cancers and chronic pancreatitis has been found. Osteonectin is expressed by a wide variety of cells, including chondrocytes, fibroblasts, platelets, endothelial cells, epithelial cells, Leydig cells, Sertoli cells, luteal cells, adrenal cortical cells, and numerous neoplastic cell lines (such as SaOS-2 cells from human osteosarcoma). Osteonectin is an acidic extracellular matrix glycoprotein that plays a vital role in bone mineralization, cell-matrix interactions, and collagen binding. Osteonectin also increases the production and activity of matrix metalloproteinases, a function important to invading cancer cells within bone. Additional functions of osteonectin beneficial to tumor cells include angiogenesis, proliferation and migration. Overexpression of osteonectin is reported in many human cancers such as breast, prostate, colon and pancreatic. This molecule has been implicated in several biological functions, including mineralization of bone and cartilage, inhibiting mineralization, modulation of cell proliferation, facilitation of acquisition of differentiated phenotype and promotion of cell attachment and spreading. A number of phosphoproteins and glycoproteins are found in bone. The phosphate is bound to the protein backbone through phosphorylated serine or threonine amino acid residues. The best characterized of these bone proteins is osteonectin. It binds collagen and hydroxyapatite in separate domains, is found in relatively large amounts in immature bone, and promotes mineralization of collagen.
- hnRNPs (make separate note)
- Heterogeneous nuclear ribonucleoproteins (hnRNPs) are complexes of RNA and protein present in the cell nucleus during gene transcription and subsequent post-transcriptional modification of the newly synthesized RNA (pre-mRNA). The presence of the proteins bound to a pre-mRNA molecule serves as a signal that the pre-mRNA is not yet fully processed and therefore not ready for export to the cytoplasm. Since most mature RNA is exported from the nucleus relatively quickly, most RNA-binding protein in the nucleus exist as heterogeneous ribonucleoprotein particles. After splicing has occurred, the proteins remain bound to spliced introns and target them for degradation. hnRNPs are also integral to the 40S subunit of the ribosome and therefore important for the translation of mRNA in the cytoplasm. However, hnRNPs also have their own nuclear localization sequences (NLS) and are therefore found mainly in the nucleus.
- Kinniburgh, A. J.; Martin, T. E. (1976-08-01). “Detection of mRNA sequences in nuclear 30S ribonucleoprotein subcomplexes”. Proceedings of the National Academy of Sciences. 73 (8): 2725–2729. Bibcode:1976PNAS…73.2725K. doi:10.1073/pnas.73.8.2725. ISSN 0027-8424. PMC 430721. PMID 1066686.
- Beyer, Ann L.; Christensen, Mark E.; Walker, Barbara W.; LeStourgeon, Wallace M. (1977). “Identification and characterization of the packaging proteins of core 40S hnRNP particles”. Cell. 11 (1): 127–138. doi:10.1016/0092-8674(77)90323-3. PMID 872217. S2CID 41245800.
- BRCA1
- hnRNP C is a key regulator of the BRCA1 and BRCA2 genes. In response to ionizing radiation, hnRNP C partially localizes to the site of DNA damage, and when depleted, S-phase progression of the cell is impaired. Additionally, BRCA1 and BRCA2 levels fall when hnRNP C is lost. BRCA1 and BRCA2 are crucial tumor-suppressor genes which are strongly implicated in breast cancers when mutated. BRCA1 in particular causes G2/M cell cycle arrest in response to DNA damage via the CHEK1 signaling cascade. hnRNP C is important for the proper expression of other tumor suppressor genes including RAD51 and BRIP1 as well. Through these genes, hnRNP is necessary to induce cell-cycle arrest in response to DNA damage by ionizing radiation.
- Martinez-Contreras, Rebeca; Cloutier, Philippe; Shkreta, Lulzim; Fisette, Jean-François; Revil, Timothée; Chabot, Benoit (2007). “HNRNP Proteins and Splicing Control”. Alternative Splicing in the Postgenomic Era. Advances in Experimental Medicine and Biology. Vol. 623. pp. 123–147. doi:10.1007/978-0-387-77374-2_8. ISBN 978-0-387-77373-5. ISSN 0065-2598. PMID 18380344.
- Anantha, Rachel W.; Alcivar, Allen L.; Ma, Jianglin; Cai, Hong; Simhadri, Srilatha; Ule, Jernej; König, Julian; Xia, Bing (2013-04-09). “Requirement of Heterogeneous Nuclear Ribonucleoprotein C for BRCA Gene Expression and Homologous Recombination”. PLOS ONE. 8 (4): e61368. Bibcode:2013PLoSO…861368A. doi:10.1371/journal.pone.0061368. ISSN 1932-6203. PMC 3621867. PMID 23585894.
- Yoshida, Kiyotsugu; Miki, Yoshio (November 2004). “Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage”. Cancer Science. 95 (11): 866–871. doi:10.1111/j.1349-7006.2004.tb02195.x. ISSN 1347-9032. PMID 15546503. S2CID 24297965.
- hnRNP C is a key regulator of the BRCA1 and BRCA2 genes. In response to ionizing radiation, hnRNP C partially localizes to the site of DNA damage, and when depleted, S-phase progression of the cell is impaired. Additionally, BRCA1 and BRCA2 levels fall when hnRNP C is lost. BRCA1 and BRCA2 are crucial tumor-suppressor genes which are strongly implicated in breast cancers when mutated. BRCA1 in particular causes G2/M cell cycle arrest in response to DNA damage via the CHEK1 signaling cascade. hnRNP C is important for the proper expression of other tumor suppressor genes including RAD51 and BRIP1 as well. Through these genes, hnRNP is necessary to induce cell-cycle arrest in response to DNA damage by ionizing radiation.
- HER2
- HER2 is overexpressed in 20-30% of breast cancers and is commonly associated with poor prognosis. It is therefore an oncogene whose differently spliced variants have been shown to have different functions. Knocking down hnRNP H1 was shown to increase the amount of an oncogenic variant Δ16HER2. HER2 is an upstream regulator of cyclin D1 and p27, and its overexpression leads to the deregulation of the G1/S checkpoint.
- Gautrey, Hannah; Jackson, Claire; Dittrich, Anna-Lena; Browell, David; Lennard, Thomas; Tyson-Capper, Alison (2015-10-03). “SRSF3 and hnRNP H1 regulate a splicing hotspot of HER2 in breast cancer cells”. RNA Biology. 12 (10): 1139–1151. doi:10.1080/15476286.2015.1076610. ISSN 1547-6286. PMC 4829299. PMID 26367347.
- Moasser, M M (2007). “The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis”. Oncogene. 26 (45): 6469–6487. doi:10.1038/sj.onc.1210477. ISSN 1476-5594. PMC 3021475. PMID 17471238.
- HER2 is overexpressed in 20-30% of breast cancers and is commonly associated with poor prognosis. It is therefore an oncogene whose differently spliced variants have been shown to have different functions. Knocking down hnRNP H1 was shown to increase the amount of an oncogenic variant Δ16HER2. HER2 is an upstream regulator of cyclin D1 and p27, and its overexpression leads to the deregulation of the G1/S checkpoint.
- p53
- hnRNPs also play a role in DNA damage response in coordination with p53. hnRNP K is rapidly induced after DNA damage by ionizing radiation. It cooperates with p53 to induce the activation of p53 target genes, thus activating cell-cycle checkpoints. p53 itself is an important tumor-suppressor gene sometimes known by the epithet “the guardian of the genome.” hnRNP K’s close association with p53 demonstrates its importance in DNA damage control. p53 regulates a large group of RNAs that are not translated into protein, called large intergenic noncoding RNAs (lincRNAs). p53 suppression of genes is often carried out by a number of these lincRNAs, which in turn have been shown to act though hnRNP K. Through physical interactions with these molecules, hnRNP K is targeted to genes and transmits p53 regulation, thus acting as a key repressor within the p53-dependent transcriptional pathway.
- Moumen, Abdeladim; Masterson, Philip; O’Connor, Mark J.; Jackson, Stephen P. (2005). “hnRNP K: An HDM2 Target and Transcriptional Coactivator of p53 in Response to DNA Damage”. Cell. 123 (6): 1065–1078. doi:10.1016/j.cell.2005.09.032. PMID 16360036. S2CID 16756766.
- Huarte, Maite; Guttman, Mitchell; Feldser, David; Garber, Manuel; Koziol, Magdalena J.; Kenzelmann-Broz, Daniela; Khalil, Ahmad M.; Zuk, Or; Amit, Ido (2010). “A Large Intergenic Noncoding RNA Induced by p53 Mediates Global Gene Repression in the p53 Response”. Cell. 142 (3): 409–419. doi:10.1016/j.cell.2010.06.040. PMC 2956184. PMID 20673990.
- Sun, Xinghui; Ali, Mohamed Sham Shihabudeen Haider; Moran, Matthew (2017-09-01). “The role of interactions of long non-coding RNAs and heterogeneous nuclear ribonucleoproteins in regulating cellular functions”. Biochemical Journal. 474 (17): 2925–2935. doi:10.1042/bcj20170280. ISSN 0264-6021. PMC 5553131. PMID 28801479.
- hnRNP serves a variety of processes in the cell, some of which include:
- Preventing the folding of pre-mRNA into secondary structures that may inhibit its interactions with other proteins.
- Possible association with the splicing apparatus.
- Transport of mRNA out of the nucleus.
- The association of a pre-mRNA molecule with a hnRNP particle prevents formation of short secondary structures dependent on base pairing of complementary regions, thereby making the pre-mRNA accessible for interactions with other proteins.
- CD44 Regulation
- hnRNP has been shown to regulate CD44, a cell-surface glycoprotein, through splicing mechanisms. CD44 is involved in cell-cell interactions and has roles in cell adhesion and migration. Splicing of CD44 and the functions of the resulting isoforms are different in breast cancer cells, and when knocked down, hnRNP reduced both cell viability and invasiveness.
- Loh, Tiing Jen; Moon, Heegyum; Cho, Sunghee; Jang, Hana; Liu, Yong Chao; Tai, Hongmei; Jung, Da-Woon; Williams, Darren R.; Kim, Hey-Ran (September 2015). “CD44 alternative splicing and hnRNP A1 expression are associated with the metastasis of breast cancer”. Oncology Reports. 34 (3): 1231–1238. doi:10.3892/or.2015.4110. ISSN 1791-2431. PMID 26151392.
- hnRNP has been shown to regulate CD44, a cell-surface glycoprotein, through splicing mechanisms. CD44 is involved in cell-cell interactions and has roles in cell adhesion and migration. Splicing of CD44 and the functions of the resulting isoforms are different in breast cancer cells, and when knocked down, hnRNP reduced both cell viability and invasiveness.
- Telomeres
- Several hnRNPs interact with telomeres, which protect the ends of chromosomes from deterioration and are often associated with cell longevity. hnRNP D associates with the G-rich repeat region of the telomeres, possibly stabilizing the region from secondary structures which would inhibit telomere replication. hnRNP has also been shown to interact with telomerase, the protein responsible for elongating telomeres and prevent their degradation. hnRNPs C1 and C2 associate with the RNA component of telomerase, which improves its ability to access the telomere.
- Eversole, A.; Maizels, N. (August 2000). “In vitro properties of the conserved mammalian protein hnRNP D suggest a role in telomere maintenance”. Molecular and Cellular Biology. 20 (15): 5425–5432. doi:10.1128/mcb.20.15.5425-5432.2000. ISSN 0270-7306. PMC 85994. PMID 10891483.
- Ford, L. P.; Suh, J. M.; Wright, W. E.; Shay, J. W. (December 2000). “Heterogeneous nuclear ribonucleoproteins C1 and C2 associate with the RNA component of human telomerase”. Molecular and Cellular Biology. 20 (23): 9084–9091. doi:10.1128/mcb.20.23.9084-9091.2000. ISSN 0270-7306. PMC 86561. PMID 11074006.
- Ford, Lance P.; Wright, Woodring E.; Shay, Jerry W. (2002-01-21). “A model for heterogeneous nuclear ribonucleoproteins in telomere and telomerase regulation”. Oncogene. 21 (4): 580–583. doi:10.1038/sj.onc.1205086. ISSN 0950-9232. PMID 11850782.
- Görlach, M.; Burd, C. G.; Dreyfuss, G. (1994-09-16). “The determinants of RNA-binding specificity of the heterogeneous nuclear ribonucleoprotein C proteins”. The Journal of Biological Chemistry. 269 (37): 23074–23078. doi:10.1016/S0021-9258(17)31621-6. ISSN 0021-9258. PMID 8083209.
- Dityatev, Alexander; El-Husseini, Alaa (2006-11-24). Molecular Mechanisms of Synaptogenesis. Springer. ISBN 9780387325620.
- Several hnRNPs interact with telomeres, which protect the ends of chromosomes from deterioration and are often associated with cell longevity. hnRNP D associates with the G-rich repeat region of the telomeres, possibly stabilizing the region from secondary structures which would inhibit telomere replication. hnRNP has also been shown to interact with telomerase, the protein responsible for elongating telomeres and prevent their degradation. hnRNPs C1 and C2 associate with the RNA component of telomerase, which improves its ability to access the telomere.
- hnRNPs also play a role in DNA damage response in coordination with p53. hnRNP K is rapidly induced after DNA damage by ionizing radiation. It cooperates with p53 to induce the activation of p53 target genes, thus activating cell-cycle checkpoints. p53 itself is an important tumor-suppressor gene sometimes known by the epithet “the guardian of the genome.” hnRNP K’s close association with p53 demonstrates its importance in DNA damage control. p53 regulates a large group of RNAs that are not translated into protein, called large intergenic noncoding RNAs (lincRNAs). p53 suppression of genes is often carried out by a number of these lincRNAs, which in turn have been shown to act though hnRNP K. Through physical interactions with these molecules, hnRNP K is targeted to genes and transmits p53 regulation, thus acting as a key repressor within the p53-dependent transcriptional pathway.
- Heterogeneous nuclear ribonucleoproteins (hnRNPs) are complexes of RNA and protein present in the cell nucleus during gene transcription and subsequent post-transcriptional modification of the newly synthesized RNA (pre-mRNA). The presence of the proteins bound to a pre-mRNA molecule serves as a signal that the pre-mRNA is not yet fully processed and therefore not ready for export to the cytoplasm. Since most mature RNA is exported from the nucleus relatively quickly, most RNA-binding protein in the nucleus exist as heterogeneous ribonucleoprotein particles. After splicing has occurred, the proteins remain bound to spliced introns and target them for degradation. hnRNPs are also integral to the 40S subunit of the ribosome and therefore important for the translation of mRNA in the cytoplasm. However, hnRNPs also have their own nuclear localization sequences (NLS) and are therefore found mainly in the nucleus.
- calreticulin
- Calreticulin also known as calregulin, CRP55, CaBP3, calsequestrin-like protein, and endoplasmic reticulum resident protein 60 (ERp60) is a protein that in humans is encoded by the CALR gene. Calreticulin is a multifunctional soluble protein that binds Ca2+ ions (a second messenger in signal transduction), rendering it inactive. The Ca2+ is bound with low affinity, but high capacity, and can be released on a signal (see inositol trisphosphate). Calreticulin is located in storage compartments associated with the endoplasmic reticulum and is considered an ER resident protein. The term “Mobilferrin” is considered to be the same as calreticulin by some sources.
- McCauliffe DP, Zappi E, Lieu TS, Michalak M, Sontheimer RD, Capra JD (Jul 1990). “A human Ro/SS-A autoantigen is the homologue of calreticulin and is highly homologous with onchocercal RAL-1 antigen and an aplysia “memory molecule””. The Journal of Clinical Investigation. 86 (1): 332–5. doi:10.1172/JCI114704. PMC 296725. PMID 2365822.
- “Entrez Gene: calreticulin“.
- Mobilferrin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Beutler E, West C, Gelbart T (June 1997). “HLA-H and associated proteins in patients with hemochromatosis”. Molecular Medicine. 3 (6): 397–402. doi:10.1007/BF03401686. PMC 2230203. PMID 9234244.
- Calreticulin binds to misfolded proteins and prevents them from being exported from the endoplasmic reticulum to the Golgi apparatus.
- A similar quality-control molecular chaperone, calnexin, performs the same service for soluble proteins as does calreticulin, however it is a membrane-bound protein. Both proteins, calnexin and calreticulin, have the function of binding to oligosaccharides containing terminal glucose residues, thereby targeting them for degradation. Calreticulin and Calnexin’s ability to bind carbohydrates associates them with the lectin protein family. In normal cellular function, trimming of glucose residues off the core oligosaccharide added during N-linked glycosylation is a part of protein processing. If “overseer” enzymes note that residues are misfolded, proteins within the rER will re-add glucose residues so that other calreticulin/calnexin can bind to these proteins and prevent them from proceeding to the Golgi. This leads these aberrantly folded proteins down a path whereby they are targeted for degradation.
- Studies on transgenic mice reveal that calreticulin is a cardiac embryonic gene that is essential during development.
- Michalak M, Lynch J, Groenendyk J, Guo L, Robert Parker JM, Opas M (Nov 2002). “Calreticulin in cardiac development and pathology”. Biochimica et Biophysica Acta (BBA) – Proteins and Proteomics. 1600 (1–2): 32–7. doi:10.1016/S1570-9639(02)00441-7. PMID 12445456.
- Calreticulin and calnexin are also integral in the production of MHC class I proteins. As newly synthesized MHC class I α-chains enter the endoplasmic reticulum, calnexin binds on to them retaining them in a partly folded state. After the β2-microglobulin binds to the peptide-loading complex (PLC), calreticulin (along with ERp57) takes over the job of chaperoning the MHC class I protein while the tapasin links the complex to the transporter associated with antigen processing (TAP) complex. This association prepares the MHC class I to bind an antigen for presentation on the cell surface.
- Murphy K (2011). Janeway’s Immunobiology (8th ed.). Oxford: Taylor & Francis. ISBN 978-0815342434.
- Transcription regulation
- Calreticulin is also found in the nucleus, suggesting that it may have a role in transcription regulation. Calreticulin binds to the synthetic peptide KLGFFKR, which is almost identical to an amino acid sequence in the DNA-binding domain of the superfamily of nuclear receptors. The amino terminus of calreticulin interacts with the DNA-binding domain of the glucocorticoid receptor and prevents the receptor from binding to its specific glucocorticoid response element. Calreticulin can inhibit the binding of androgen receptor to its hormone-responsive DNA element and can inhibit androgen receptor and retinoic acid receptor transcriptional activities in vivo, as well as retinoic acid-induced neuronal differentiation. Thus, calreticulin can act as an important modulator of the regulation of gene transcription by nuclear hormone receptors.
- Clinical significance
- Calreticulin binds to antibodies in certain area of systemic lupus and Sjögren patients that contain anti-Ro/SSA antibodies. Systemic lupus erythematosus is associated with increased autoantibody titers against calreticulin, but calreticulin is not a Ro/SS-A antigen. Earlier papers referred to calreticulin as an Ro/SS-A antigen, but this was later disproven. Increased autoantibody titer against human calreticulin is found in infants with complete congenital heart block of both the IgG and IgM classes.
- In 2013, two groups detected calreticulin mutations in a majority of JAK2-negative/MPL-negative patients with essential thrombocythemia and primary myelofibrosis, which makes CALR mutations the second most common in myeloproliferative neoplasms. All mutations (insertions or deletions) affected the last exon, generating a reading frame shift of the resulting protein, that creates a novel terminal peptide and causes a loss of endoplasmic reticulum KDEL retention signal.
- Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, Avezov E, Li J, Kollmann K, Kent DG, Aziz A, Godfrey AL, Hinton J, Martincorena I, Van Loo P, Jones AV, Guglielmelli P, Tarpey P, Harding HP, Fitzpatrick JD, Goudie CT, Ortmann CA, Loughran SJ, Raine K, Jones DR, Butler AP, Teague JW, O’Meara S, McLaren S, Bianchi M, Silber Y, Dimitropoulou D, Bloxham D, Mudie L, Maddison M, Robinson B, Keohane C, Maclean C, Hill K, Orchard K, Tauro S, Du MQ, Greaves M, Bowen D, Huntly BJ, Harrison CN, Cross NC, Ron D, Vannucchi AM, Papaemmanuil E, Campbell PJ, Green AR (Dec 2013). “Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2”. The New England Journal of Medicine. 369 (25): 2391–405. doi:10.1056/NEJMoa1312542. PMC 3966280. PMID 24325359.
- Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, Them NC, Berg T, Gisslinger B, Pietra D, Chen D, Vladimer GI, Bagienski K, Milanesi C, Casetti IC, Sant’Antonio E, Ferretti V, Elena C, Schischlik F, Cleary C, Six M, Schalling M, Schönegger A, Bock C, Malcovati L, Pascutto C, Superti-Furga G, Cazzola M, Kralovics R (Dec 2013). “Somatic mutations of calreticulin in myeloproliferative neoplasms”. The New England Journal of Medicine. 369 (25): 2379–90. doi:10.1056/NEJMoa1311347. PMID 24325356.
- Role in cancer
- Calreticulin (CRT) is expressed in many cancer cells and plays a role to promote macrophages to engulf hazardous cancerous cells. The reason why most of the cells are not destroyed is the presence of another molecule with signal CD47, which blocks CRT. Hence antibodies that block CD47 might be useful as a cancer treatment. In mice models of myeloid leukemia and non-Hodgkin lymphoma, anti-CD47 were effective in clearing cancer cells while normal cells were unaffected.
- Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB, Raveh T, Park CY, Majeti R, Weissman IL (Dec 2010). “Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47”. Science Translational Medicine. 2 (63): 63ra94. doi:10.1126/scitranslmed.3001375. PMC4126904. PMID21178137.
- Christopher Vaughan (December 22, 2010). “Many cancer cells found to have an ‘eat me’ signal in study”. Stanford School of Medicine. Archived from the original on 2013-10-16.
- Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB, Raveh T, Park CY, Majeti R, Weissman IL (Dec 2010). “Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47”. Science Translational Medicine. 2 (63): 63ra94. doi:10.1126/scitranslmed.3001375. PMC4126904. PMID21178137.
- Calreticulin (CRT) is expressed in many cancer cells and plays a role to promote macrophages to engulf hazardous cancerous cells. The reason why most of the cells are not destroyed is the presence of another molecule with signal CD47, which blocks CRT. Hence antibodies that block CD47 might be useful as a cancer treatment. In mice models of myeloid leukemia and non-Hodgkin lymphoma, anti-CD47 were effective in clearing cancer cells while normal cells were unaffected.
- Calreticulin has been shown to interact with Perforin and NK2 homeobox 1.
- Andrin C, Pinkoski MJ, Burns K, Atkinson EA, Krahenbuhl O, Hudig D, Fraser SA, Winkler U, Tschopp J, Opas M, Bleackley RC, Michalak M (Jul 1998). “Interaction between a Ca2+-binding protein calreticulin and perforin, a component of the cytotoxic T-cell granules”. Biochemistry. 37 (29): 10386–94. doi:10.1021/bi980595z. PMID 9671507.
- Perrone L, Tell G, Di Lauro R (Feb 1999). “Calreticulin enhances the transcriptional activity of thyroid transcription factor-1 by binding to its homeodomain”. The Journal of Biological Chemistry. 274 (8): 4640–5. doi:10.1074/jbc.274.8.4640. PMID 9988700.
- Calreticulin also known as calregulin, CRP55, CaBP3, calsequestrin-like protein, and endoplasmic reticulum resident protein 60 (ERp60) is a protein that in humans is encoded by the CALR gene. Calreticulin is a multifunctional soluble protein that binds Ca2+ ions (a second messenger in signal transduction), rendering it inactive. The Ca2+ is bound with low affinity, but high capacity, and can be released on a signal (see inositol trisphosphate). Calreticulin is located in storage compartments associated with the endoplasmic reticulum and is considered an ER resident protein. The term “Mobilferrin” is considered to be the same as calreticulin by some sources.
- cubilin
- Cubilin is a protein that in humans is encoded by the CUBN gene. Cubilin (CUBN) acts as a receptor for intrinsic factor-vitamin B12 complexes. The role of receptor is supported by the presence of 27 CUB domains. Cubilin shows a restricted mode of expression according to protein profiling and transcriptomics analyses, and is essentially only present in the kidneys and small intestine. Mutations in CUBN may play a role in autosomal recessive megaloblastic anemia. A complex of amnionless and cubilin forms the cubam receptor.
- Kozyraki R, Kristiansen M, Silahtaroglu A, Hansen C, Jacobsen C, Tommerup N, Verroust PJ, Moestrup SK (Jun 1998). “The human intrinsic factor-vitamin B12 receptor, cubilin: molecular characterization and chromosomal mapping of the gene to 10p within the autosomal recessive megaloblastic anemia (MGA1) region”. Blood. 91 (10): 3593–600. doi:10.1182/blood.V91.10.3593. PMID 9572993.
- Moestrup SK, Kozyraki R, Kristiansen M, Kaysen JH, Rasmussen HH, Brault D, Pontillon F, Goda FO, Christensen EI, Hammond TG, Verroust PJ (Mar 1998). “The intrinsic factor-vitamin B12 receptor and target of teratogenic antibodies is a megalin-binding peripheral membrane protein with homology to developmental proteins”. J Biol Chem. 273 (9): 5235–42. doi:10.1074/jbc.273.9.5235. PMID 9478979.
- “Entrez Gene: CUBN cubilin (intrinsic factor-cobalamin receptor)“.
- Uhlén, Mathias; Fagerberg, Linn; Hallström, Björn M.; Lindskog, Cecilia; Oksvold, Per; Mardinoglu, Adil; Sivertsson, Åsa; Kampf, Caroline; Sjöstedt, Evelina (2015-01-23). “Tissue-based map of the human proteome”. Science. 347 (6220): 1260419. doi:10.1126/science.1260419. ISSN 0036-8075. PMID 25613900. S2CID 802377.
- “Tissue expression of CUBN – Summary – The Human Protein Atlas”. www.proteinatlas.org. Retrieved 2017-09-06.
- Clinical significance
- Cubilin is a potential diagnostic and prognostic cancer biomarker for kidney cancer. Based on patient survival data, high levels of cubilin in tumor cells is a favourable prognostic biomarker in renal cell carcinoma.
- Gremel, Gabriela; Djureinovic, Dijana; Niinivirta, Marjut; Laird, Alexander; Ljungqvist, Oscar; Johannesson, Henrik; Bergman, Julia; Edqvist, Per-Henrik; Navani, Sanjay (2017-01-04). “A systematic search strategy identifies cubilin as independent prognostic marker for renal cell carcinoma”. BMC Cancer. 17 (1): 9. doi:10.1186/s12885-016-3030-6. ISSN 1471-2407. PMC 5215231. PMID 28052770.
- Uhlen, Mathias; Zhang, Cheng; Lee, Sunjae; Sjöstedt, Evelina; Fagerberg, Linn; Bidkhori, Gholamreza; Benfeitas, Rui; Arif, Muhammad; Liu, Zhengtao (2017-08-18). “A pathology atlas of the human cancer transcriptome”. Science. 357 (6352): eaan2507. doi:10.1126/science.aan2507. ISSN 0036-8075. PMID 28818916.
- “Expression of CUBN in renal cancer – The Human Protein Atlas”. www.proteinatlas.org. Retrieved 2017-09-06.
- Cubilin is a potential diagnostic and prognostic cancer biomarker for kidney cancer. Based on patient survival data, high levels of cubilin in tumor cells is a favourable prognostic biomarker in renal cell carcinoma.
- Cubilin is a protein that in humans is encoded by the CUBN gene. Cubilin (CUBN) acts as a receptor for intrinsic factor-vitamin B12 complexes. The role of receptor is supported by the presence of 27 CUB domains. Cubilin shows a restricted mode of expression according to protein profiling and transcriptomics analyses, and is essentially only present in the kidneys and small intestine. Mutations in CUBN may play a role in autosomal recessive megaloblastic anemia. A complex of amnionless and cubilin forms the cubam receptor.
- megalin.
- Low density lipoprotein receptor-related protein 2 also known as LRP-2 or megalin is a protein which in humans is encoded by the LRP2gene. LRP2 was identified as the antigen of rat experimental membranous nephropathy (Heyman nephritis) and originally named gp330 and subsequently megalin and later LRP2. LRP2/megalin is a multiligand binding receptor found in the plasma membrane of many absorptive epithelial cells. LRP2 is an approximately 600kDa (4665 amino acids) transmembrane glycoprotein with structural similarities to the low density lipoprotein receptor (LDLR). LRP2 has a NPXY motif that is the binding site for Dab2 to initiate clathrin-mediated endocytosis. LRP2 forms a homodimer that changes conformation in response to pH. At pH 7.5 (extracellular pH), LRP2 is considered active, with the leucine loops in an open conformation to allow ligands to bind. At acidic endosomal pHs, the leucine loops collapse to prevent ligands binding.
- “Entrez Gene: LRP2 low density lipoprotein-related protein 2“.
- Korenberg JR, Argraves KM, Chen XN, Tran H, Strickland DK, Argraves WS (July 1994). “Chromosomal localization of human genes for the LDL receptor family member glycoprotein 330 (LRP2) and its associated protein RAP (LRPAP1)”. Genomics. 22 (1): 88–93. doi:10.1006/geno.1994.1348. PMID 7959795.
- Farquhar MG (September 1995). “The unfolding story of megalin (gp330): now recognized as a drug receptor”. The Journal of Clinical Investigation. 96 (3): 1184. doi:10.1172/JCI118149. PMC 185736. PMID 7657789.
- Farquhar MG, Saito A, Kerjaschki D, Orlando RA (July 1995). “The Heymann nephritis antigenic complex: megalin (gp330) and RAP”. Journal of the American Society of Nephrology. 6 (1): 35–47. doi:10.1681/ASN.V6135. PMID 7579068.
- Eshbach ML, Weisz OA (February 2017). “Receptor-Mediated Endocytosis in the Proximal Tubule”. Annual Review of Physiology. 79 (1): 425–448. doi:10.1146/annurev-physiol-022516-034234. PMC 5512543. PMID 27813828.
- Gallagher H, Oleinikov AV, Fenske C, Newman DJ (March 2004). “The adaptor disabled-2 binds to the third psi xNPxY sequence on the cytoplasmic tail of megalin”. Biochimie. 86 (3): 179–182. doi:10.1016/j.biochi.2004.03.001. PMID 15134832.
- Beenken A, Cerutti G, Brasch J, Guo Y, Sheng Z, Erdjument-Bromage H, et al. (February 2023). “Structures of LRP2 reveal a molecular machine for endocytosis”. Cell. 186 (4): 821–836.e13. doi:10.1016/j.cell.2023.01.016. PMC 9993842. PMID 36750096.
- LRP2 is expressed in epithelial cells of the thyroid (thyrocytes), where it can serve as a receptor for the protein thyroglobulin (Tg). LRP2 is also expressed on the apical surface of epithelial cells in the proximal tubule of the kidney. It is highly expressed in the first segment (S1) of the proximal tubule, with decreasing expression in the second (S2) and third segment (S3) of the proximal tubule. LRP2 is also expressed in podocytes, and antigenic response to LRP2 in podocytes is the primary cause of Heymann nephritis in rats.
- Farquhar MG, Saito A, Kerjaschki D, Orlando RA (July 1995). “The Heymann nephritis antigenic complex: megalin (gp330) and RAP”. Journal of the American Society of Nephrology. 6 (1): 35–47. doi:10.1681/ASN.V6135. PMID 7579068.
- Eshbach ML, Weisz OA (February 2017). “Receptor-Mediated Endocytosis in the Proximal Tubule”. Annual Review of Physiology. 79 (1): 425–448. doi:10.1146/annurev-physiol-022516-034234. PMC 5512543. PMID 27813828.
- Zheng G, Marino’ M, Zhao J, McCluskey RT (March 1998). “Megalin (gp330): a putative endocytic receptor for thyroglobulin (Tg)”. Endocrinology. 139 (3): 1462–1465. doi:10.1210/endo.139.3.5978. PMID 9492085.
- LRP2/megalin functions to mediate endocytosis of ligands leading to degradation in lysosomes or transcytosis. LRP2/megalin can also form complexes with CUBAM, the cubilin and amnionless complex. Those complexes are able to reabsorb several molecules and can be inhibited by sodium maleate. LRP2 and CUBAM are responsible for the uptake of most of the filtered proteins that escape the glomerular filtration barrier in the proximal tubule of the kidney. The endocytic capacity of the proximal tubule cells is dictated by the combined function of LRP2, CUBAM, and Dab2.
- Weisz OA (July 2021). “Endocytic adaptation to functional demand by the kidney proximal tubule”. The Journal of Physiology. 599 (14): 3437–3446. doi:10.1113/JP281599. PMC 8715547. PMID 34036593.
- Long KR, Rbaibi Y, Bondi CD, Ford BR, Poholek AC, Boyd-Shiwarski CR, et al. (January 2022). “Cubilin-, megalin-, and Dab2-dependent transcription revealed by CRISPR/Cas9 knockout in kidney proximal tubule cells”. American Journal of Physiology. Renal Physiology. 322 (1): F14–F26. doi:10.1152/ajprenal.00259.2021. PMC 8698540. PMID 34747197.
- The epithelial cells of the proximal tubule are highly polarized and have a robust apical endocytic pathway, subapical compartmentalization, and large endocytic capacity. This pathway is mediated by LRP2 and CUBAM, where Dab2 binds to the cytoplasmic tails of both LRP2 and CUBAM to initiate clathrin-coated endocytosis. Once internalized, the endosomes release their clathrin coats and fuse with a dense subapical network of tubules to recycle receptors back to the apical surface. As the endosomes acidify, LRP2 release its cargo and undergoes a conformational change which collapses the binding pockets to inhibit ligands rebinding to LRP2 in the endosomes. Recycling of the LRP2 occurs from apical vacuoles with Rab11a positive endosomes, also referred to as dense apical tubules. The vesicles are directed back to the plasma membrane where LRP2 undergoes another conformational change due to the change in pH and becomes active again. According to LRP2/megalin kinetic modeling, the rate of megalin recycling and return to the apical surface from dense apical tubules has the largest impact on determining the overall endocytic capacity of proximal tubule cells and the endocytic rate of LRP2. The fraction of LRP2 at the apical surface is important for the continued ability of the protein to reabsorb filtered proteins in the proximal tubule to maintain the robust endocytic capacity of these cells.
- Weisz OA (July 2021). “Endocytic adaptation to functional demand by the kidney proximal tubule”. The Journal of Physiology. 599 (14): 3437–3446. doi:10.1113/JP281599. PMC 8715547. PMID 34036593.
- Long KR, Rbaibi Y, Bondi CD, Ford BR, Poholek AC, Boyd-Shiwarski CR, et al. (January 2022). “Cubilin-, megalin-, and Dab2-dependent transcription revealed by CRISPR/Cas9 knockout in kidney proximal tubule cells”. American Journal of Physiology. Renal Physiology. 322 (1): F14–F26. doi:10.1152/ajprenal.00259.2021. PMC 8698540. PMID 34747197.
- Shipman KE, Long KR, Cowan IA, Rbaibi Y, Baty CJ, Weisz OA (2022-10-28). “An Adaptable Physiological Model of Endocytic Megalin Trafficking in Opossum Kidney Cells and Mouse Kidney Proximal Tubule”. Function. 3 (6): zqac046. doi:10.1093/function/zqac046. PMC 9614980. PMID 36325513.
- Eshbach ML, Weisz OA (February 2017). “Receptor-Mediated Endocytosis in the Proximal Tubule”. Annual Review of Physiology. 79 (1): 425–448. doi:10.1146/annurev-physiol-022516-034234. PMC 5512543. PMID 27813828.
- Beenken A, Cerutti G, Brasch J, Guo Y, Sheng Z, Erdjument-Bromage H, et al. (February 2023). “Structures of LRP2 reveal a molecular machine for endocytosis”. Cell. 186 (4): 821–836.e13. doi:10.1016/j.cell.2023.01.016. PMC 9993842. PMID 36750096.
- Clinical significance
- Disfunction in the LRP2-mediated endocytic trafficking and endocytic capacity in the proximal tubule can result in low molecular weight proteinuria, which is a hallmark of many diseases.
- Weisz OA (July 2021). “Endocytic adaptation to functional demand by the kidney proximal tubule”. The Journal of Physiology. 599 (14): 3437–3446. doi:10.1113/JP281599. PMC 8715547. PMID 34036593
- Mutations in the LRP2 gene are associated with Donnai-Barrow syndrome.
- Kantarci S, Al-Gazali L, Hill RS, Donnai D, Black GC, Bieth E, et al. (August 2007). “Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes”. Nature Genetics. 39 (8): 957–959. doi:10.1038/ng2063. PMC 2891728. PMID 17632512.
- Dent’s Disease (Dent 1) is associated with a drop in LRP2/megalin protein level in the proximal tubule with no detectable decrease in mRNA, suggesting that the loss of ClC-5, the gene mutated in Dent’s Disease, shortens the half-life of the LRP2 receptor. The loss of ClC-5 has been found to delay the early endosome maturation in the LRP2 trafficking in the proximal tubule cells.
- Shipman KE, Weisz OA (2020-09-14). “Making a Dent in Dent Disease”. Function. 1 (2): zqaa017. doi:10.1093/function/zqaa017. PMC 7519470. PMID 33015630.
- Shipman KE, Baty CJ, Long KR, Rbaibi Y, Cowan IA, Gerges M, et al. (April 2023). “Impaired Endosome Maturation Mediates Tubular Proteinuria in Dent Disease Cell Culture and Mouse Models”. Journal of the American Society of Nephrology. 34 (4): 619–640. doi:10.1681/ASN.0000000000000084. PMC 10103310. PMID 36758125. S2CID 256737627.
- A decrease in LRP2 receptor expression has been reported in animal models of acute and chronic kidney diseases. LRP2 has been shown to play a role in the development of nephrotoxic acute kidney injury (AKI) by mediating the uptake of nephrotoxic agents. However, there have been no further studies to show the functional importance of LRP2 or CUBAM in the progression of AKI.
- Nielsen R, Christensen EI, Birn H (January 2016). “Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease”. Kidney International. 89 (1): 58–67. doi:10.1016/j.kint.2015.11.007. PMID 26759048.
- LRP2 has been shown to associate with the following proteins in the plasma membrane/cytosol of cells:
- LRP2 has been shown to bind to the following ligands:
- Disfunction in the LRP2-mediated endocytic trafficking and endocytic capacity in the proximal tubule can result in low molecular weight proteinuria, which is a hallmark of many diseases.
- Low density lipoprotein receptor-related protein 2 also known as LRP-2 or megalin is a protein which in humans is encoded by the LRP2gene. LRP2 was identified as the antigen of rat experimental membranous nephropathy (Heyman nephritis) and originally named gp330 and subsequently megalin and later LRP2. LRP2/megalin is a multiligand binding receptor found in the plasma membrane of many absorptive epithelial cells. LRP2 is an approximately 600kDa (4665 amino acids) transmembrane glycoprotein with structural similarities to the low density lipoprotein receptor (LDLR). LRP2 has a NPXY motif that is the binding site for Dab2 to initiate clathrin-mediated endocytosis. LRP2 forms a homodimer that changes conformation in response to pH. At pH 7.5 (extracellular pH), LRP2 is considered active, with the leucine loops in an open conformation to allow ligands to bind. At acidic endosomal pHs, the leucine loops collapse to prevent ligands binding.
- Merlot AM, Kalinowski DS, Richardson DR (2014). “Unraveling the mysteries of serum albumin-more than just a serum protein”. Frontiers in Physiology. 5: 299. doi:10.3389/fphys.2014.00299. PMC 4129365. PMID 25161624.
In medicine and pharmacology, albondin (gp60) is a cell receptor that binds serum albumin. It seems to be expressed on endothelial cells and binding induces endocytosis. Not much is known about this protein, except for its approximate molecular mass of 60 kDa.
- Schnitzer, J. E.; Oh, P. (1994). “Albondin-mediated capillary permeability to albumin. Differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins”. The Journal of Biological Chemistry. 269 (8): 6072–6082. doi:10.1016/S0021-9258(17)37571-3. PMID 8119952.
- ^ Merlot, AM; Kalinowski, DS; Richardson, DR (2014). “Unraveling the mysteries of serum albumin-more than just a serum protein”. Frontiers in Physiology. 5: 299. doi:10.3389/fphys.2014.00299. PMC 4129365. PMID 25161624.
See also
References
- GRCh38: Ensembl release 89: ENSG00000163631 – Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000029368 – Ensembl, May 2017
- “Human PubMed Reference:”. National Center for Biotechnology Information, U.S. National Library of Medicine.
- “Mouse PubMed Reference:”. National Center for Biotechnology Information, U.S. National Library of Medicine.
- “Harmonisation of Reference Intervals” (PDF). pathologyharmony.co.uk. Pathology Harmony. Archived from the original (PDF) on 2 August 2013. Retrieved 23 June 2013.
- “Hypoalbuminemia: Background, Pathophysiology, Etiology”. Medscape Reference. 2019-11-10. Retrieved 2019-12-22.
- Kouchakzadeh H, Shojaosadati SA, Shokri F (September 2014). “Efficient loading and entrapment of tamoxifen in human serum albumin based nanoparticulate delivery system by a modified desolvation technique”. Chemical Engineering Research and Design. 92 (9): 1681–1692. doi:10.1016/j.cherd.2013.11.024.
- di Masi A, Leboffe L, Polticelli F, Tonon F, Zennaro C, Caterino M, et al. (September 2018). “Human Serum Albumin Is an Essential Component of the Host Defense Mechanism Against Clostridium difficile Intoxication”. The Journal of Infectious Diseases. 218 (9): 1424–1435. doi:10.1093/infdis/jiy338. PMID 29868851.
- “Albumin: analyte monograph” (PDF). Association for Clinical Biochemistry and Laboratory Medicine. Archived from the original (PDF) on 13 November 2012. Retrieved 23 June 2013.
- “Normal Ranges for Common Laboratory Tests”. Archived from the original on 2013-01-14. Retrieved 2007-12-06. Rush University
- Pasantes-Morales H, Wright CE, Gaull GE (December 1984). “Protective effect of taurine, zinc and tocopherol on retinol-induced damage in human lymphoblastoid cells”. The Journal of Nutrition. 114 (12): 2256–2261. doi:10.1093/jn/114.12.2256. PMID 6502269.
- Masaki T, Matsuura T, Ohkawa K, Miyamura T, Okazaki I, Watanabe T, Suzuki T (July 2006). “All-trans retinoic acid down-regulates human albumin gene expression through the induction of C/EBPbeta-LIP”. The Biochemical Journal. 397 (2): 345–353. doi:10.1042/BJ20051863. PMC 1513275. PMID 16608438.
- Anderson DM (2000). Dorland’s illustrated medical dictionary (29th ed.). Philadelphia [u.a.]: Saunders. p. 860. ISBN 978-0721682617.
- Zerbato V, Sanson G, De Luca M, Di Bella S, di Masi A, Caironi P, et al. (2022-04-20). “The Impact of Serum Albumin Levels on COVID-19 Mortality”. Infectious Disease Reports. 14 (3): 278–286. doi:10.3390/idr14030034. ISSN 2036-7449. PMC 9149867. PMID 35645213.
- Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, et al. (September 2012). “The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience”. JACC. Cardiovascular Interventions. 5 (9): 974–981. doi:10.1016/j.jcin.2012.06.011. PMC 3717525. PMID 22995885.
- Uthamalingam S, Kandala J, Daley M, Patvardhan E, Capodilupo R, Moore SA, Januzzi JL (December 2010). “Serum albumin and mortality in acutely decompensated heart failure”. American Heart Journal. 160 (6): 1149–1155. doi:10.1016/j.ahj.2010.09.004. PMID 21146671.
- Xu R, Hao M, Zhou W, Liu M, Wei Y, Xu J, Zhang W (August 2022). “Preoperative hypoalbuminemia in patients undergoing cardiac surgery: a meta-analysis”. Surgery Today. 53 (8): 861–872. doi:10.1007/s00595-022-02566-9. PMID 35933630. S2CID 251369303.
- Zerbato V, Sanson G, De Luca M, Di Bella S, di Masi A, Caironi P, et al. (April 2022). “The Impact of Serum Albumin Levels on COVID-19 Mortality”. Infectious Disease Reports. 14 (3): 278–286. doi:10.3390/idr14030034. PMC 9149867. PMID 35645213.
- Busher JT (1990). “Chapter 101: Serum Albumin and Globulin”. In Walker HK, Hall WD, Hurst JW (eds.). Clinical methods : the history, physical, and laboratory examinations (3rd ed.). Boston: Butterworths. ISBN 978-0409900774. PMID 21250048.
- Mutlu EA, Keshavarzian A, Mutlu GM (June 2006). “Hyperalbuminemia and elevated transaminases associated with high-protein diet”. Scandinavian Journal of Gastroenterology. 41 (6): 759–760. doi:10.1080/00365520500442625. PMID 16716979. S2CID 21264934.
- Roberts I, Blackhall K, Alderson P, Bunn F, Schierhout G (November 2011). “Human albumin solution for resuscitation and volume expansion in critically ill patients”. The Cochrane Database of Systematic Reviews. 2011 (11): CD001208. doi:10.1002/14651858.CD001208.pub4. hdl:2299/5243. PMC 7055200. PMID 22071799.
- Yu YT, Liu J, Hu B, Wang RL, Yang XH, Shang XL, et al. (July 2021). “Expert consensus on the use of human serum albumin in critically ill patients”. Chinese Medical Journal. 134 (14): 1639–1654. doi:10.1097/CM9.0000000000001661. PMC 8318641. PMID 34397592.
- Lo AH, Kripfgans OD, Carson PL, Rothman ED, Fowlkes JB (May 2007). “Acoustic droplet vaporization threshold: effects of pulse duration and contrast agent”. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 54 (5): 933–946. doi:10.1109/tuffc.2007.339. PMID 17523558. S2CID 11983041.
- Ascenzi P, Leboffe L, Toti D, Polticelli F, Trezza V (August 2018). “Fipronil recognition by the FA1 site of human serum albumin”. Journal of Molecular Recognition. 31 (8): e2713. doi:10.1002/jmr.2713. PMID 29656610. S2CID 4894574.
- Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, et al. (June 2018). “Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial”. Lancet. 391 (10138): 2417–2429. doi:10.1016/S0140-6736(18)30840-7. hdl:2108/208667. PMID 29861076. S2CID 44120418.
- Rahbar S (October 1968). “An abnormal hemoglobin in red cells of diabetics”. Clinica Chimica Acta; International Journal of Clinical Chemistry. 22 (2): 296–298. doi:10.1016/0009-8981(68)90372-0. PMID 5687098.
- Day JF, Thorpe SR, Baynes JW (February 1979). “Nonenzymatically glucosylated albumin. In vitro preparation and isolation from normal human serum”. The Journal of Biological Chemistry. 254 (3): 595–597. doi:10.1016/S0021-9258(17)37845-6. PMID 762083.
- Iberg N, Flückiger R (October 1986). “Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites”. The Journal of Biological Chemistry. 261 (29): 13542–13545. doi:10.1016/S0021-9258(18)67052-8. PMID 3759977.
- Jakus V, Hrnciarová M, Cársky J, Krahulec B, Rietbrock N (1999). “Inhibition of nonenzymatic protein glycation and lipid peroxidation by drugs with antioxidant activity”. Life Sciences. 65 (18–19): 1991–1993. doi:10.1016/S0024-3205(99)00462-2. PMID 10576452.
- Mohamadi-Nejad A, Moosavi-Movahedi AA, Hakimelahi GH, Sheibani N (September 2002). “Thermodynamic analysis of human serum albumin interactions with glucose: insights into the diabetic range of glucose concentration”. The International Journal of Biochemistry & Cell Biology. 34 (9): 1115–1124. doi:10.1016/S1357-2725(02)00031-6. PMID 12009306.
- Shaklai N, Garlick RL, Bunn HF (March 1984). “Nonenzymatic glycosylation of human serum albumin alters its conformation and function”. The Journal of Biological Chemistry. 259 (6): 3812–3817. doi:10.1016/S0021-9258(17)43168-1. PMID 6706980.
- Mendez DL, Jensen RA, McElroy LA, Pena JM, Esquerra RM (December 2005). “The effect of non-enzymatic glycation on the unfolding of human serum albumin”. Archives of Biochemistry and Biophysics. 444 (2): 92–99. doi:10.1016/j.abb.2005.10.019. PMID 16309624.
- Mohamadi-Nejada A, Moosavi-Movahedi AA, Safariana S, Naderi-Maneshc MH, Ranjbarc B, Farzamid B, Mostafavie H, Larijanif MB, Hakimelahi GH (July 2002). “The thermal analysis of nonezymatic glycosylation of human serum albumin: differential scanning calorimetry and circular dichroism studies”. Thermochimica Acta. 389 (1–2): 141–151. doi:10.1016/S0040-6031(02)00006-0.
- Kańska U, Boratyński J (2002). “Thermal glycation of proteins by D-glucose and D-fructose”. Archivum Immunologiae et Therapiae Experimentalis. 50 (1): 61–66. PMID 11916310.
- Rojas A, Romay S, González D, Herrera B, Delgado R, Otero K (February 2000). “Regulation of endothelial nitric oxide synthase expression by albumin-derived advanced glycosylation end products”. Circulation Research. 86 (3): E50–E54. doi:10.1161/01.RES.86.3.e50. PMID 10679490.
- Garlick RL, Mazer JS (May 1983). “The principal site of nonenzymatic glycosylation of human serum albumin in vivo”. The Journal of Biological Chemistry. 258 (10): 6142–6146. doi:10.1016/S0021-9258(18)32384-6. PMID 6853480.
- Kawakami A, Kubota K, Yamada N, Tagami U, Takehana K, Sonaka I, et al. (July 2006). “Identification and characterization of oxidized human serum albumin. A slight structural change impairs its ligand-binding and antioxidant functions”. The FEBS Journal. 273 (14): 3346–3357. doi:10.1111/j.1742-4658.2006.05341.x. PMID 16857017. S2CID 12844381.
- Turell L, Carballal S, Botti H, Radi R, Alvarez B (April 2009). “Oxidation of the albumin thiol to sulfenic acid and its implications in the intravascular compartment”. Brazilian Journal of Medical and Biological Research = Revista Brasileira de Pesquisas Medicas e Biologicas. 42 (4): 305–311. doi:10.1590/s0100-879×2009000400001. PMID 19330257.
- Rosas-Díaz M, Camarillo-Cadena M, Hernández-Arana A, Ramón-Gallegos E, Medina-Navarro R (June 2015). “Antioxidant capacity and structural changes of human serum albumin from patients in advanced stages of diabetic nephropathy and the effect of the dialysis”. Molecular and Cellular Biochemistry. 404 (1–2): 193–201. doi:10.1007/s11010-015-2378-2. PMID 25758354. S2CID 6718332.
- Watanabe H, Imafuku T, Otagiri M, Maruyama T (2017). “Clinical Implications Associated With the Posttranslational Modification-Induced Functional Impairment of Albumin in Oxidative”. Journal of Pharmaceutical Sciences. 106 (9): 2195–2203. doi:10.1016/j.xphs.2017.03.002. PMID 28302542.
- Matsuyama Y, Terawaki H, Terada T, Era S (August 2009). “Albumin thiol oxidation and serum protein carbonyl formation are progressively enhanced with advancing stages of chronic kidney disease”. Clinical and Experimental Nephrology. 13 (4): 308–315. doi:10.1007/s10157-009-0161-y. PMID 19363646. S2CID 20886185.
- “Microalbumin Urine Test”. WebMD.
- Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL (February 2003). “The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan”. The Journal of Experimental Medicine. 197 (3): 315–322. doi:10.1084/jem.20021829. PMC 2193842. PMID 12566415.
- Merlot AM, Kalinowski DS, Richardson DR (2014). “Unraveling the mysteries of serum albumin-more than just a serum protein”. Frontiers in Physiology. 5: 299. doi:10.3389/fphys.2014.00299. PMC 4129365. PMID 25161624.
Further reading
- Komatsu T, Nakagawa A, Curry S, Tsuchida E, Murata K, Nakamura N, Ohno H (September 2009). “The role of an amino acid triad at the entrance of the heme pocket in human serum albumin for O(2) and CO binding to iron protoporphyrin IX”. Organic & Biomolecular Chemistry. 7 (18): 3836–3841. doi:10.1039/b909794e. PMID 19707690.
- Milojevic J, Raditsis A, Melacini G (November 2009). “Human serum albumin inhibits Abeta fibrillization through a “monomer-competitor” mechanism”. Biophysical Journal. 97 (9): 2585–2594. Bibcode:2009BpJ….97.2585M. doi:10.1016/j.bpj.2009.08.028. PMC 2770600. PMID 19883602.
- Silva AM, Hider RC (October 2009). “Influence of non-enzymatic post-translation modifications on the ability of human serum albumin to bind iron. Implications for non-transferrin-bound iron speciation”. Biochimica et Biophysica Acta. 1794 (10): 1449–1458. doi:10.1016/j.bbapap.2009.06.003. PMID 19505594.
- Otosu T, Nishimoto E, Yamashita S (February 2010). “Multiple conformational state of human serum albumin around single tryptophan residue at various pH revealed by time-resolved fluorescence spectroscopy”. Journal of Biochemistry. 147 (2): 191–200. doi:10.1093/jb/mvp175. PMID 19884191.
- Blindauer CA, Harvey I, Bunyan KE, Stewart AJ, Sleep D, Harrison DJ, et al. (August 2009). “Structure, properties, and engineering of the major zinc binding site on human albumin”. The Journal of Biological Chemistry. 284 (34): 23116–23124. doi:10.1074/jbc.M109.003459. PMC 2755717. PMID 19520864.
- Juárez J, López SG, Cambón A, Taboada P, Mosquera V (July 2009). “Influence of electrostatic interactions on the fibrillation process of human serum albumin”. The Journal of Physical Chemistry B. 113 (30): 10521–10529. doi:10.1021/jp902224d. PMID 19572666.
- Fu BL, Guo ZJ, Tian JW, Liu ZQ, Cao W (August 2009). “[Advanced glycation end products induce expression of PAI-1 in cultured human proximal tubular epithelial cells through NADPH oxidase dependent pathway]”. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi = Chinese Journal of Cellular and Molecular Immunology. 25 (8): 674–677. PMID 19664386.
- Ascenzi P, di Masi A, Coletta M, Ciaccio C, Fanali G, Nicoletti FP, et al. (November 2009). “Ibuprofen impairs allosterically peroxynitrite isomerization by ferric human serum heme-albumin”. The Journal of Biological Chemistry. 284 (45): 31006–31017. doi:10.1074/jbc.M109.010736. PMC 2781501. PMID 19734142.
- Sowa ME, Bennett EJ, Gygi SP, Harper JW (July 2009). “Defining the human deubiquitinating enzyme interaction landscape”. Cell. 138 (2): 389–403. doi:10.1016/j.cell.2009.04.042. PMC 2716422. PMID 19615732.
- Curry S (August 2002). “Beyond expansion: structural studies on the transport roles of human serum albumin”. Vox Sanguinis. 83 (Suppl 1): 315–319. doi:10.1111/j.1423-0410.2002.tb05326.x. PMID 12617161. S2CID 44482133.
- Guo S, Shi X, Yang F, Chen L, Meehan EJ, Bian C, Huang M (September 2009). “Structural basis of transport of lysophospholipids by human serum albumin”. The Biochemical Journal. 423 (1): 23–30. doi:10.1042/BJ20090913. PMID 19601929.
- de Jong PE, Gansevoort RT (2009). “Focus on microalbuminuria to improve cardiac and renal protection”. Nephron Clinical Practice. 111 (3): c204-10, discussion c211. doi:10.1159/000201568. PMID 19212124.
- Page TA, Kraut ND, Page PM, Baker GA, Bright FV (September 2009). “Dynamics of loop 1 of domain I in human serum albumin when dissolved in ionic liquids”. The Journal of Physical Chemistry B. 113 (38): 12825–12830. doi:10.1021/jp904475v. PMID 19711930.
- Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E (June 2008). “The antioxidant properties of serum albumin”. FEBS Letters. 582 (13): 1783–1787. doi:10.1016/j.febslet.2008.04.057. PMID 18474236. S2CID 5364683.
- Wyatt AR, Wilson MR (February 2010). “Identification of human plasma proteins as major clients for the extracellular chaperone clusterin”. The Journal of Biological Chemistry. 285 (6): 3532–3539. doi:10.1074/jbc.M109.079566. PMC 2823492. PMID 19996109.
- Cui FL, Yan YH, Zhang QZ, Qu GR, Du J, Yao XJ (February 2010). “A study on the interaction between 5-Methyluridine and human serum albumin using fluorescence quenching method and molecular modeling”. Journal of Molecular Modeling. 16 (2): 255–262. doi:10.1007/s00894-009-0548-4. PMID 19588173. S2CID 9042021.
- Caridi G, Dagnino M, Simundic AM, Miler M, Stancic V, Campagnoli M, et al. (March 2010). “Albumin Benkovac (c.1175 A > G; p.Glu392Gly): a novel genetic variant of human serum albumin”. Translational Research. 155 (3): 118–119. doi:10.1016/j.trsl.2009.10.001. PMID 20171595.
- Deeb O, Rosales-Hernández MC, Gómez-Castro C, Garduño-Juárez R, Correa-Basurto J (February 2010). “Exploration of human serum albumin binding sites by docking and molecular dynamics flexible ligand-protein interactions”. Biopolymers. 93 (2): 161–170. doi:10.1002/bip.21314. PMID 19785033.
- Karahan SC, Koramaz I, Altun G, Uçar U, Topbaş M, Menteşe A, Kopuz M (2010). “Ischemia-modified albumin reduction after coronary bypass surgery is associated with the cardioprotective efficacy of cold-blood cardioplegia enriched with N-acetylcysteine: a preliminary study”. European Surgical Research. 44 (1): 30–36. doi:10.1159/000262324. PMID 19955769. S2CID 26699371.
- Jin C, Lu L, Zhang RY, Zhang Q, Ding FH, Chen QJ, Shen WF (October 2009). “Association of serum glycated albumin, C-reactive protein and ICAM-1 levels with diffuse coronary artery disease in patients with type 2 diabetes mellitus”. Clinica Chimica Acta; International Journal of Clinical Chemistry. 408 (1–2): 45–49. doi:10.1016/j.cca.2009.07.003. PMID 19615354.
External links
- Human Albumin structure in the Protein data bank
- Human Serum Albumin Archived 2006-04-24 at the Wayback Machine on the Human Protein Reference Database Archived 2006-04-24 at the Wayback Machine
- Albumin binding prediction
- Albumin at Lab Tests Online
- Albumin: analyte monograph from the Association for Clinical Biochemistry and Laboratory Medicine
- Overview of all the structural information available in the PDB for UniProt: P02768 (Serum albumin) at the PDBe-KB.


Leave a Reply