
Graft-versus-host disease (GvHD) is divided into acute and chronic forms
Graft-versus-host disease (GvHD) is a syndrome, characterized by inflammation in different organs. GvHD is commonly associated with bone marrow transplants and stem cell transplants.
Not to be confused with Host-versus-graft disease.
White blood cells of the donor’s immune system which remain within the donated tissue (the graft) recognize the recipient (the host) as foreign (non-self). The white blood cells present within the transplanted tissue then attack the recipient’s body’s cells, which leads to GvHD. This should not be confused with a transplant rejection, which occurs when the immune system of the transplant recipient rejects the transplanted tissue; GvHD occurs when the donor’s immune system’s white blood cells reject the recipient. The underlying principle (alloimmunity) is the same, but the details and course may differ.
GvHD can also occur after a blood transfusion, known as Transfusion-associated graft-versus-host disease or TA-GvHD if the blood products used have not been gamma irradiated or treated with an approved leukocyte reduction system. In contrast to organ/tissue transplant associated GvHD, the incidence of TA-GvHD is increased with HLA matching (first-degree or close relatives).
- Williamson, Lorna M. (1998-09-01). “Transfusion associated graft versus host disease and its prevention”. Heart. 80 (3): 211–212. doi:10.1136/hrt.80.3.211. ISSN 1355-6037. PMC 1761088. PMID 9875072.
Types
In the clinical setting, graft-versus-host disease is divided into acute and chronic forms, and scored or graded on the basis of the tissue affected and the severity of the reaction.
- Martino R, Romero P, Subirá M, Bellido M, Altés A, Sureda A, et al. (August 1999). “Comparison of the classic Glucksberg criteria and the IBMTR Severity Index for grading acute graft-versus-host disease following HLA-identical sibling stem cell transplantation. International Bone Marrow Transplant Registry”. Bone Marrow Transplantation. 24 (3): 283–7. doi:10.1038/sj.bmt.1701899. PMID 10455367. S2CID 24811357.
- Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. (December 2005). “National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report”. Biology of Blood and Marrow Transplantation. 11 (12): 945–56. doi:10.1016/j.bbmt.2005.09.004. PMC 4329079. PMID 16338616.
In the classical sense, acute graft-versus-host disease is characterized by selective damage to the liver, skin (rash), mucosa, and the gastrointestinal tract. Newer research indicates that other graft-versus-host disease target organs include the immune system (the hematopoietic system, e.g., the bone marrow and the thymus) itself, and the lungs in the form of immune-mediated pneumonitis. Biomarkers can be used to identify specific causes of GvHD, such as elafin in the skin. Chronic graft-versus-host disease also attacks the above organs, but over its long-term course can also cause damage to the connective tissue and exocrine glands.
- Morisse-Pradier H, Nove-Josserand R, Philit F, Senechal A, Berger F, Callet-Bauchu E, et al. (February 2016). “[Graft-versus-host disease, a rare complication of lung transplantation]”. Revue de Pneumologie Clinique. 72 (1): 101–7. doi:10.1016/j.pneumo.2015.05.004. PMID 26209034.
- Paczesny S, Braun TM, Levine JE, Hogan J, Crawford J, Coffing B, et al. (January 2010). “Elafin is a biomarker of graft-versus-host disease of the skin”. Science Translational Medicine. 2 (13): 13–14. doi:10.1016/j.bbmt.2008.12.039. PMC 2895410. PMID 20371463.
- Ogawa Y, Shimmura S, Dogru M, Tsubota K (November 2010). “Immune processes and pathogenic fibrosis in ocular chronic graft-versus-host disease and clinical manifestations after allogeneic hematopoietic stem cell transplantation”. Cornea. 29 Suppl 1 (Nov Supplement 1): S68-77. doi:10.1097/ICO.0b013e3181ea9a6b. PMID 20935546. S2CID 39209313.
Elafin, also known as peptidase inhibitor 3 or skin-derived antileukoprotease (SKALP), is a protein that in humans is encoded by the PI3 gene. This gene encodes an elastase-specific protease inhibitor, which contains a WAP-type four-disulfide core (WFDC) domain, and is thus a member of the WFDC domain family. Most WFDC gene members are localized to chromosome 20q12-q13 in two clusters: centromeric and telomeric. This gene belongs to the centromeric cluster. Elafin has been found to have utility in serving as a biomarker for graft versus host disease of the skin. Elafin plays some role in gut inflammation.
- GRCh38: Ensembl release 89: ENSG00000124102 – Ensembl, May 2017
- “Human PubMed Reference:”. National Center for Biotechnology Information, U.S. National Library of Medicine.
- Molhuizen HO, Zeeuwen PL, Olde Weghuis D, Geurts van Kessel A, Schalkwijk J (Feb 1994). “Assignment of the human gene encoding the epidermal serine proteinase inhibitor SKALP (PI3) to chromosome region 20q12→q13”. Cytogenet Cell Genet. 66 (2): 129–31. doi:10.1159/000133683. PMID 8287685.
- Clauss A, Lilja H, Lundwall A (Nov 2002). “A locus on human chromosome 20 contains several genes expressing protease inhibitor domains with homology to whey acidic protein”. Biochem J. 368 (Pt 1): 233–42. doi:10.1042/BJ20020869. PMC 1222987. PMID 12206714.
- “Entrez Gene: PI3 peptidase inhibitor 3, skin-derived (SKALP)”.
- Paczesny S, Levine JE, Hogan J, Crawford J, Braun TM, Wang H, Faca V, Zhang Q, Pitteri S, Chin A, Choi SW, Kitko CL, Krijanovski OI, Reddy P, Mineishi S, Whitfield J, Jones S, Hanash SM, Ferrara JLM (February 2009). “[Elafin is a Biomarker of Graft Versus Host Disease of the Skin”. Biology of Blood and Marrow Transplantation. 15 (2 Suppl 1): 13–14. doi:10.1016/j.bbmt.2008.12.039. PMC 2895410. PMID 20371463.
- “Archives”. Los Angeles Times. November 2012.
Mucosal damage to the vagina can result in severe pain and scarring, and appears in both acute and chronic GvHD. This can result in an inability to have sexual intercourse.
- Spiryda LB, Laufer MR, Soiffer RJ, Antin JA (December 2003). “Graft-versus-host disease of the vulva and/or vagina: diagnosis and treatment”. Biology of Blood and Marrow Transplantation. 9 (12): 760–5. doi:10.1016/j.bbmt.2003.08.001. PMID 14677115.
Acute
The acute or fulminant form of the disease (aGvHD) is normally observed within the first 10 to 100 days post-transplant, and is a major challenge to transplants owing to associated morbidity and mortality. About one-third to one-half of allogeneic transplant recipients will develop acute GvHD. It is less common in younger patients and in those with closer human leukocyte antigens (HLA) matches between donor and the patient.
- Funke VA, Moreira MC, Vigorito AC (October 2016). “Acute and chronic Graft-versus-host disease after hematopoietic stem cell transplantation”. Revista da Associação Médica Brasileira. 62 (supl 1): 44–50. doi:10.1590/1806-9282.62.suppl1.44. PMID 27982319.
- “Stem Cell or Bone Marrow Transplant Side Effects”. www.cancer.org. Retrieved 2020-09-01.
- Goker H, Haznedaroglu IC, Chao NJ (March 2001). “Acute graft-vs-host disease: pathobiology and management”. Experimental Hematology. 29 (3): 259–77. doi:10.1016/S0301-472X(00)00677-9. PMID 11274753.
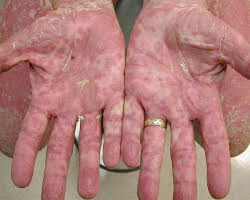
The first signs are usually a rash, burning, and redness of the skin on the palms and soles. This can spread over the entire body. Other symptoms can include nausea, vomiting, stomach cramps, diarrhea (watery and sometimes bloody), loss of appetite, jaundice, abdominal pain, and weight loss.
- “Stem Cell or Bone Marrow Transplant Side Effects”. www.cancer.org. Retrieved 2020-09-01.
Acute GvHD of the GI tract can result in severe intestinal inflammation, sloughing of the mucosal membrane, severe diarrhea, abdominal pain, nausea, and vomiting. This is typically diagnosed via intestinal biopsy. Liver GvHD is measured by the bilirubin level in acute patients. Skin GvHD results in a diffuse red maculopapular rash, sometimes in a lacy pattern.
- “Graft-versus-host disease”. MedlinePlus. National Library of Medicine. Retrieved 6 May 2019.
- Krejci M, Kamelander J, Pospisil Z, Mayer J (2012). “Kinetics of bilirubin and liver enzymes is useful for predicting of liver graft-versus-host disease”. Neoplasma. 59 (3): 264–8. doi:10.4149/neo_2012_034. PMID 22296496.
- Feito-Rodríguez M, de Lucas-Laguna R, Gómez-Fernández C, Sendagorta-Cudós E, Collantes E, Beato MJ, Boluda ER (2013). “Cutaneous graft versus host disease in pediatric multivisceral transplantation”. Pediatric Dermatology. 30 (3): 335–41. doi:10.1111/j.1525-1470.2012.01839.x. PMID 22957989. S2CID 25151282.
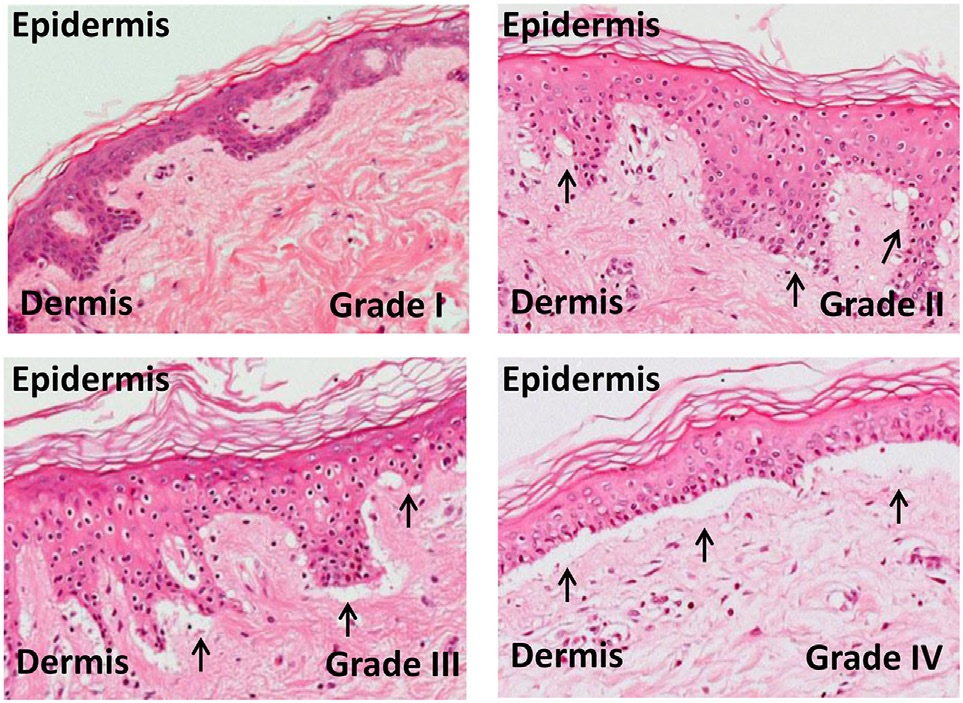
Acute GvHD is staged as follows: overall grade (skin-liver-gut) with each organ staged individually from a low of 1 to a high of 4. Patients with grade IV GvHD usually have a poor prognosis. If the GvHD is severe and requires intense immunosuppression involving steroids and additional agents to get under control, the patient may develop severe infections as a result of the immunosuppression and may die of infection. However, a 2016 study found that the prognosis for patients with grade IV GvHD has improved in recent years.
- “Graft-versus-host disease”. MedlinePlus. National Library of Medicine. Retrieved 6 May 2019.
- El-Jawahri A, Li S, Antin JH, Spitzer TR, Armand PA, Koreth J, et al. (May 2016). “Improved Treatment-Related Mortality and Overall Survival of Patients with Grade IV Acute GVHD in the Modern Years”. Biology of Blood and Marrow Transplantation. 22 (5): 910–8. doi:10.1016/j.bbmt.2015.12.024. PMID 26748160.
Chronic
The chronic form of graft-versus-host disease (cGvHD) normally begins 90 to 600 days post-transplant. The appearance of moderate to severe cases of cGVHD adversely influences long-term survival.
- Lee SJ, Vogelsang G, Flowers ME (April 2003). “Chronic graft-versus-host disease”. Biology of Blood and Marrow Transplantation. 9 (4): 215–33. doi:10.1053/bbmt.2003.50026. PMID 12720215.
- “Stem Cell or Bone Marrow Transplant Side Effects”. www.cancer.org. Retrieved 2020-09-01.
The first symptom of cGvHD is commonly a rash on the palms of the hands or the soles of the feet, and the rash can spread and is usually itchy and dry. In severe cases, the skin may blister and peel, like a bad sunburn. A fever may also develop.
Other symptoms of chronic GVHD can include:
- Decreased appetite
- Diarrhea
- Abdominal (belly) cramps
- Weight loss
- Yellowing of the skin and eyes (jaundice)
- Enlarged liver
- Bloated abdomen (belly)
- Pain in the upper right part of the abdomen (belly)
- Increased levels of liver enzymes in the blood (seen on blood tests)
- Skin that feels tight
- Dry, burning eyes
- Dryness or painful sores in the mouth
- Burning sensations when eating acidic foods
- Bacterial infections
- Blockages in the smaller airways of the lungs
In the oral cavity, chronic graft-versus-host disease manifests as lichen planus with a higher risk of malignant transformation to oral squamous cell carcinoma in comparison to the classical oral lichen planus. Oral cancer associated with graft-versus-host disease may have more aggressive behavior with poorer prognosis, when compared to oral cancer in non-hematopoietic stem cell transplantation patients.
- El-Jawahri A, Li S, Antin JH, Spitzer TR, Armand PA, Koreth J, et al. (May 2016). “Improved Treatment-Related Mortality and Overall Survival of Patients with Grade IV Acute GVHD in the Modern Years”. Biology of Blood and Marrow Transplantation. 22 (5): 910–8. doi:10.1016/j.bbmt.2015.12.024. PMID 26748160.
- Tsukada S, Itonaga H, Taguchi J, Miyoshi T, Hayashida S, Sato S, et al. (2019). “[Gingival squamous cell carcinoma diagnosed on the occasion of osteonecrosis of the jaw in a patient with chronic GVHD]”. [Rinsho Ketsueki] the Japanese Journal of Clinical Hematology. 60 (1): 22–27. doi:10.11406/rinketsu.60.22. PMID 30726819.
Causes
Three criteria, known as the Billingham criteria, must be met in order for GvHD to occur.
- An immuno-competent graft is administered, with viable and functional immune cells.
- The recipient is immunologically different from the donor – histo-incompatible.
- The recipient is immunocompromised and therefore cannot destroy or inactivate the transplanted cells. In particular, it involves an inability of the recipient’s cell-mediated immunity to destroy or inactivate viable lymphocytes from the donor.
- Yasuda H, Ohto H, Abe R (1993). “Mechanism of transfusion-associated graft-versus-host disease”. Fukushima J Med Sci. 39 (2): 69–75. PMID 7927137.
- Billingham RE (1966). “The biology of graft-versus-host reactions”. Harvey Lectures. 62 (62): 21–78. PMID 4875305.
After bone marrow transplantation, T cells present in the graft, either as contaminants or intentionally introduced into the host, attack the tissues of the transplant recipient after perceiving host tissues as antigenically foreign. The T cells produce an excess of cytokines, including TNF-α and interferon-gamma (IFNγ). A wide range of host antigens can initiate graft-versus-host disease, among them the human leukocyte antigens (HLA). However, graft-versus-host disease can occur even when HLA-identical siblings are the donors. HLA-identical siblings or HLA-identical unrelated donors often have genetically different proteins (called minor histocompatibility antigens) that can be presented by major histocompatibility complex (MHC) molecules to the donor’s T-cells, which see these antigens as foreign and so mount an immune response.
- Kanda J (September 2013). “Effect of HLA mismatch on acute graft-versus-host disease”. International Journal of Hematology. 98 (3): 300–8. doi:10.1007/s12185-013-1405-x. PMID 23893313. S2CID 1585777.
- Bonifazi F, Solano C, Wolschke C, Sessa M, Patriarca F, Zallio F, et al. (February 2019). “Acute GVHD prophylaxis plus ATLG after myeloablative allogeneic haemopoietic peripheral blood stem-cell transplantation from HLA-identical siblings in patients with acute myeloid leukaemia in remission: final results of quality of life and long-term outcome analysis of a phase 3 randomised study”. The Lancet. Haematology. 6 (2): e89–e99. doi:10.1016/S2352-3026(18)30214-X. hdl:10138/311714. PMID 30709437. S2CID 73449161.
- Taylor CJ, Bolton EM, Bradley JA (August 2011). “Immunological considerations for embryonic and induced pluripotent stem cell banking”. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 366 (1575): 2312–22. doi:10.1098/rstb.2011.0030. PMC 3130422. PMID 21727137.
Antigens most responsible for graft loss are HLA-DR (first six months), HLA-B (first two years), and HLA-A (long-term survival).
- Solomon S, Pitossi F, Rao MS (February 2015). “Banking on iPSC–is it doable and is it worthwhile”. Stem Cell Reviews and Reports. 11 (1): 1–10. doi:10.1007/s12015-014-9574-4. PMC 4333229. PMID 25516409.
While donor T-cells are undesirable as effector cells of graft-versus-host disease, they are valuable for engraftment by preventing the recipient’s residual immune system from rejecting the bone marrow graft (host-versus-graft). In addition, as bone marrow transplantation is frequently used to treat cancer, mainly leukemias, donor T-cells have proven to have a valuable graft-versus-tumor effect. A great deal of current research on allogeneic bone marrow transplantation involves attempts to separate the undesirable graft-vs-host disease aspects of T-cell physiology from the desirable graft-versus-tumor effect.
- Falkenburg JH, Jedema I (December 2017). “Graft versus tumor effects and why people relapse”. Hematology. American Society of Hematology. Education Program. 2017 (1): 693–698. doi:10.1182/asheducation-2017.1.693. PMC 6142614. PMID 29222323.
- Sun K, Li M, Sayers TJ, Welniak LA, Murphy WJ (August 2008). “Differential effects of donor T-cell cytokines on outcome with continuous bortezomib administration after allogeneic bone marrow transplantation”. Blood. 112 (4): 1522–9. doi:10.1182/blood-2008-03-143461. PMC 2515132. PMID 18539902.
Transfusion-associated GvHD
Main article: Transfusion-associated graft versus host disease
This type of GvHD is associated with transfusion of un-irradiated blood to immunocompromised recipients. It can also occur in situations in which the blood donor is homozygous and the recipient is heterozygous for an HLA haplotype. It is associated with higher mortality (80–90%) due to involvement of bone marrow lymphoid tissue, however the clinical manifestations are similar to GVHD resulting from bone marrow transplantation. Transfusion-associated GvHD is rare in modern medicine. It is almost entirely preventable by controlled irradiation of blood products to inactivate the white blood cells (including lymphocytes) within.
- Moroff G, Leitman SF, Luban NL (October 1997). “Principles of blood irradiation, dose validation, and quality control”. Transfusion. 37 (10): 1084–92. doi:10.1046/j.1537-2995.1997.371098016450.x. PMID 9354830. S2CID 7462268.
Transfusion-associated graft-versus-host disease (TA-GvHD) is a rare complication of blood transfusion, in which the immunologically competent donor T lymphocytes mount an immune response against the recipient’s lymphoid tissue. These donor lymphocytes engraft, recognize recipient cells as foreign and mount an immune response against recipient tissues. Donor lymphocytes are usually identified as foreign and destroyed by the recipient’s immune system. However, in situations where the recipient is severely immunocompromised, or when the donor and recipient HLA type is similar (as can occur in directed donations from first-degree relatives), the recipient’s immune system is not able to destroy the donor lymphocytes. This can result in transfusion associated graft-versus-host disease. This is in contrast with organ/tissue transplant associated GvHD, where matching HLA reduces the incident of the complication. The clinical presentation is the same as GvHD occurring in other settings, such as bone marrow transplantation. TA-GvHD can develop two days to six weeks after the transfusion.
Typical symptoms include:
- fever
- erythematous maculopapular rash, which can progress to generalised erythroderma
- toxic epidermal necrolysis in extreme cases
- hepatomegaly
- diarrhea
Other symptoms can include cough, abdominal pain, dyspnea and vomiting.
Laboratory findings include pancytopenia, marrow aplasia, abnormal liver enzymes, and electrolyte imbalance (when diarrhea is present).[citation needed] TA-GvHD can be suspected from a biopsy of the affected skin or liver, and established by HLA analysis of the circulating lymphocytes. This testing can identify circulating lymphocytes with a different HLA type than the tissue cells of the host.[citation needed]
Prevention includes gamma irradiation of the lymphocyte-containing blood components such as red blood cells, platelets and granulocytes. Irradiated blood components should be issued in the following situations:
- Intrauterine transfusions
- Prematurity, low birthweight, or erythroblastosis fetalis in newborns
- Congenital immunodeficiencies
- Certain hematologic malignancies (e.g. Hodgkin lymphoma)
- Patients undergoing hematopoietic stem cell transplantation
- Components that are HLA matched, or directed donations from a family member
- Patients receiving fludarabine therapy
- Patients receiving granulocyte transfusions
Treatment is supportive. No available form of therapy has proven effective in treating TA-GvHD and it is fatal in more than 90% of cases. The most common causes of death in TA-GvHD are infections and hemorrhages secondary to pancytopenia and liver dysfunction.[citation needed]
The incidence of TA-GvHD in immunocompromised patients receiving blood transfusions is estimated to be 0.1–1.0%, and mortality around 80–90%. Mortality is higher in TA-GvHD than in GvHD associated with bone marrow transplantation, where the engrafted lymphoid cells in the bone marrow are of donor origin (in autotransplant) and therefore the immune reaction is not directed against them.[citation needed]
In 2023, the first case of fetal-induced GvHD was reported in the New England Journal of Medicine.
- “Complications of Transfusion: Transfusion Medicine: Merck Manual Professional”. Retrieved 2009-02-09.
- Savage WJ (June 2016). “Transfusion Reactions”. Hematology/Oncology Clinics of North America. 30 (3): 619–634. doi:10.1016/j.hoc.2016.01.012. PMID 27113000.
- Vaillant AA, Modi P, Mohammadi O (2022). “Graft Versus Host Disease”. StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 30855823. Retrieved 2023-02-02.
- “National Healthcare Safety Network (NHSN)”. www.cdc.gov. 2017-12-29. Retrieved 2018-09-18.
- Patel KK, Patel AK, Ranjan RR, Shah AP (September 2010). “Transfusion associated graft versus host disease following whole blood transfusion from an unrelated donor in an immunocompetent patient”. Indian Journal of Hematology & Blood Transfusion. 26 (3): 92–95. doi:10.1007/s12288-010-0028-0. PMC 3002081. PMID 21886390.
- A Case of Fetal-Induced Graft-versus-Host Disease
- Fung MK, Grossman BJ, Hillyer CD, Westhoff CM (2014). Technical manual (18th ed.). Bethesda, Md.: American Association of Blood Banks. ISBN 978-1563958885. OCLC 881812415.
Thymus transplantation
Thymus transplantation may be said to be able to cause a special type of GvHD because the recipient’s thymocytes would use the donor thymus cells as models when going through the negative selection to recognize self-antigens, and could therefore still mistake own structures in the rest of the body for being non-self. This is a rather indirect GvHD because it is not directly cells in the graft itself that causes it but cells in the graft that make the recipient’s T cells act like donor T cells. It can be seen as a multiple-organ autoimmunity in xenotransplantation experiments of the thymus between different species. Autoimmune disease is a frequent complication after human allogeneic thymus transplantation, found in 42% of subjects over one year post-transplantation. However, this is partially explained by the fact that the indication itself, that is, complete DiGeorge syndrome, increases the risk of autoimmune disease.
- Xia G, Goebels J, Rutgeerts O, Vandeputte M, Waer M (February 2001). “Transplantation tolerance and autoimmunity after xenogeneic thymus transplantation”. Journal of Immunology. 166 (3): 1843–54. doi:10.4049/jimmunol.166.3.1843. PMID 11160231. S2CID 24007739.
- Markert ML, Devlin BH, McCarthy EA, Chinn IK, Hale LP (2008). “Thymus Transplantation”. In Lavini C, Moran CA, Morandi U, et al. (eds.). Thymus Gland Pathology: Clinical, Diagnostic, and Therapeutic Features. pp. 255–267. doi:10.1007/978-88-470-0828-1_30. ISBN 978-88-470-0827-4. S2CID 219575147.
- Markert ML, Devlin BH, Alexieff MJ, Li J, McCarthy EA, Gupton SE, et al. (May 2007). “Review of 54 patients with complete DiGeorge anomaly enrolled in protocols for thymus transplantation: outcome of 44 consecutive transplants”. Blood. 109 (10): 4539–47. doi:10.1182/blood-2006-10-048652. PMC 1885498. PMID 17284531.
Thymoma-associated multiorgan autoimmunity (TAMA)
A GvHD-like disease called thymoma-associated multiorgan autoimmunity (TAMA) can occur in patients with thymoma. In these patients rather than a donor being a source of pathogenic T cells, the patient’s own malignant thymus produces self-directed T cells. This is because the malignant thymus is incapable of appropriately educating developing thymocytes to eliminate self-reactive T cells. The result is a disease virtually indistinguishable from GvHD.
- Wadhera A, Maverakis E, Mitsiades N, Lara PN, Fung MA, Lynch PJ (October 2007). “Thymoma-associated multiorgan autoimmunity: a graft-versus-host-like disease”. Journal of the American Academy of Dermatology. 57 (4): 683–9. doi:10.1016/j.jaad.2007.02.027. PMID 17433850.
Thymoma-associated multiorgan autoimmunity (TAMA) is a severe often fatal disease that presents in some patients with thymoma. It has also been referred to in the medical literature as “thymoma-associated graft-versus-host-like disease“. Patients with TAMA present with variable combinations of a morbilliform skin eruption, chronic diarrhea, and abnormal liver enzymes. The histopathology of the skin, liver, or bowel mucosa resembles GVHD.
Thymoma is a common neoplasm arising from the thymus, the primary lymphoid organ where T cells become educated to distinguish “self” from “non self”. In the setting of thymoma, abnormal thymic education occurs as a result of subtle differences in antigen processing. In TAMA these differences result in autoreactive T cells escaping from the thymus. This results in a condition similar to graft-versus-host disease.
Patients often have a refractory disease course but some patients may respond to phototherapy.
This disease name was coined by Emanual Maverakis and described in detail in 2007 but case reports of graft-versus-host-like disease in the setting of thymoma date back to at least the mid 1990s.
- Wadhera A, Maverakis E, Mitsiades N, Lara PN, Fung MA, Lynch PJ (Oct 2007). “Thymoma-associated multiorgan autoimmunity: a graft-versus-host-like disease”. J Am Acad Dermatol. 57 (4): 683–9. doi:10.1016/j.jaad.2007.02.027. PMID 17433850.
- Meneshian A, Giaccone G, Olivier KR. “Clinical presentation and management of thymoma and thymic carcinoma”. UpToDate.
- Nakayama M, Itoh M, Kikuchi S, Tanito K, Nakagawa H (Apr 2016). “Thymoma-associated cutaneous graft-versus-host-like disease possibly treated with Narrow-band UVB phototherapy”. Eur J Dermatol. 26 (2): 208–9. doi:10.1684/ejd.2015.2716. PMID 27018042. S2CID 26959211.
- Kornacki S, Hansen FC, Lazenby A (1995). “Graft-versus-host-like colitis associated with malignant thymoma”. Am J Surg Pathol. 19 (2): 224–8. doi:10.1097/00000478-199502000-00011. PMID 7832281.
Mechanism
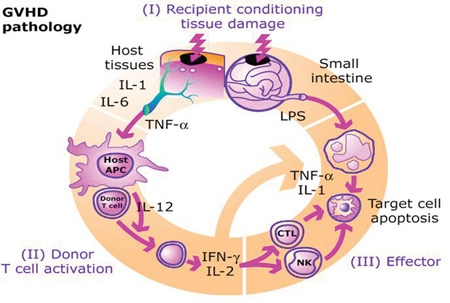
The pathophysiology of GvHD includes three phases:
- The afferent phase: activation of APC (antigen presenting cells)
- The efferent phase: activation, proliferation, differentiation and migration of effector cells
- The effector phase: target tissue destruction
- Nassereddine S, Rafei H, Elbahesh E, Tabbara I (April 2017). “Versus Host Disease: A Comprehensive Review”. Anticancer Research. 37 (4): 1547–1555. doi:10.21873/anticanres.11483. PMID 28373413.
Activation of APC occurs in the first stage of GvHD. Prior to haematopoietic stem cell transplantation, radiation or chemotherapy results in damage and activation of host tissues, especially intestinal mucosa. This allows the microbial products to enter and stimulate pro-inflammatory cytokines such as IL-1 and TNF-α. These proinflammatory cytokines increase the expression of MHC and adhesion molecules on APCs, thereby increasing the ability of APC to present antigen. The second phase is characterized by the activation of effector cells. Activation of donor T-cells further enhances the expression of MHC and adhesion molecules, chemokines and the expansion of CD8 + and CD4 + T-cells and guest B-cells. In the final phase, these effector cells migrate to target organs and mediate tissue damage, resulting in multiorgan failure.
- Roncarolo MG, Battaglia M (August 2007). “Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans”. Nature Reviews. Immunology. 7 (8): 585–98. doi:10.1038/nri2138. PMID 17653126. S2CID 7043844.
- Zhang L, Chu J, Yu J, Wei W (February 2016). “Cellular and molecular mechanisms in graft-versus-host disease”. Journal of Leukocyte Biology. 99 (2): 279–87. doi:10.1189/jlb.4ru0615-254rr. PMID 26643713. S2CID 25250676.
Prevention
- DNA-based tissue typing allows for more precise HLA matching between donors and transplant patients, which has been proven to reduce the incidence and severity of GvHD and to increase long-term survival.
- Morishima Y, Sasazuki T, Inoko H, Juji T, Akaza T, Yamamoto K, et al. (June 2002). “The clinical significance of human leukocyte antigen (HLA) allele compatibility in patients receiving a marrow transplant from serologically HLA-A, HLA-B, and HLA-DR matched unrelated donors”. Blood. 99 (11): 4200–6. doi:10.1182/blood.V99.11.4200. PMID 12010826. S2CID 6859250.
- The T-cells of umbilical cord blood (UCB) have an inherent immunological immaturity, and the use of UCB stem cells in unrelated donor transplants has a reduced incidence and severity of GvHD.
- Grewal SS, Barker JN, Davies SM, Wagner JE (June 2003). “Unrelated donor hematopoietic cell transplantation: marrow or umbilical cord blood?”. Blood. 101 (11): 4233–44. doi:10.1182/blood-2002-08-2510. PMID 12522002. S2CID 6486524.
- Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE, et al. (June 2001). “Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors”. The New England Journal of Medicine. 344 (24): 1815–22. doi:10.1056/NEJM200106143442402. PMID 11407342.
- Methotrexate, cyclosporin and tacrolimus are common drugs used for GvHD prophylaxis. Further research is necessary to evaluate whether mesenchymal stromal cells can also be used for the prophylaxis.
- Törlén J, Ringdén O, Garming-Legert K, Ljungman P, Winiarski J, Remes K, et al. (November 2016). “A prospective randomized trial comparing cyclosporine/methotrexate and tacrolimus/sirolimus as graft-versus-host disease prophylaxis after allogeneic hematopoietic stem cell transplantation”. Haematologica. 101 (11): 1417–1425. doi:10.3324/haematol.2016.149294. PMC 5394879. PMID 27662016.
- Fisher SA, Cutler A, Doree C, Brunskill SJ, Stanworth SJ, Navarrete C, Girdlestone J, et al. (Cochrane Haematological Malignancies Group) (January 2019). “Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition”. The Cochrane Database of Systematic Reviews. 1 (1): CD009768. doi:10.1002/14651858.CD009768.pub2. PMC 6353308. PMID 30697701.
- Graft-versus-host disease can largely be avoided by performing a T-cell-depleted bone marrow transplant. However, these types of transplants come at a cost of diminished graft-versus-tumor effect, greater risk of engraftment failure, or cancer relapse, and general immunodeficiency, resulting in a patient more susceptible to viral, bacterial, and fungal infection. In a multi-center study, disease-free survival at 3 years was not different between T cell-depleted and T cell-replete transplants.
- Hale G, Waldmann H (May 1994). “Control of graft-versus-host disease and graft rejection by T cell depletion of donor and recipient with Campath-1 antibodies. Results of matched sibling transplants for malignant diseases”. Bone Marrow Transplantation. 13 (5): 597–611. PMID 8054913.
- Wagner JE, Thompson JS, Carter SL, Kernan NA (2005). “Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II-III trial”. Lancet. 366 (9487): 733–41. doi:10.1016/S0140-6736(05)66996-6. PMID 16125590. S2CID 33732870.
Treatment
Glucocorticoids
Intravenously administered glucocorticoids, such as prednisone, are the standard of care in acute GvHD and chronic GVHD.
- Goker H, Haznedaroglu IC, Chao NJ (March 2001). “Acute graft-vs-host disease: pathobiology and management”. Experimental Hematology. 29 (3): 259–77. doi:10.1016/S0301-472X(00)00677-9. PMID 11274753.
- Menillo SA, Goldberg SL, McKiernan P, Pecora AL (October 2001). “Intraoral psoralen ultraviolet A irradiation (PUVA) treatment of refractory oral chronic graft-versus-host disease following allogeneic stem cell transplantation”. Bone Marrow Transplantation. 28 (8): 807–8. doi:10.1038/sj.bmt.1703231. PMID 11781637. S2CID 27292769.
The use of these glucocorticoids is designed to suppress the T-cell-mediated immune onslaught on the host tissues; however, in high doses, this immune-suppression raises the risk of infections and cancer relapse. Therefore, it is desirable to taper off the post-transplant high-level steroid doses to lower levels, at which point the appearance of mild GVHD may be welcome, especially in HLA mis-matched patients, as it is typically associated with a graft-versus-tumor effect.[citation needed].
While glucocorticoids remain the first line of treatment for acute GVHD, only about 50% of patients respond to treatment, otherwise having steroid-refractory GVHD (SR-GVHD). An increasing number of recent treatment options for SR-GVHD have been investigated, such as extracorporeal photopheresis (ECP), mesenchymal stem cell (MSCs), fecal microbial transplantation (FMT), and the medication Ruxolitinib.
- Flinn, Aisling M; Gennery, Andrew R (2023-03-06). “Recent advances in graft-versus-host disease”. Faculty Reviews. 12: 4. doi:10.12703/r/12-4. ISSN 2732-432X. PMC 10009889. PMID 36923700.
Steroid-sparing immunosuppression/immunomodulation
Cyclosporine and tacrolimus are calcineurin inhibitors. The substances are structurally different but have the same mechanism of action. Cyclosporine binds to the cytosolic protein peptidyl-prolyl cis-trans isomerase A (known as cyclophilin), while tacrolimus binds to the cytosolic protein peptidyl-prolyl cis-trans isomerase FKBP12. These complexes inhibit calcineurin, block dephosphorylation of the transcription factor NFAT of activated T-cells and its translocation into the nucleus. Standard prophylaxis involves the use of cyclosporine for six months with methotrexate. Cyclosporin levels should be maintained above 200 ng/ml.
- Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL (August 1991). “Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes”. Cell. 66 (4): 807–15. doi:10.1016/0092-8674(91)90124-h. PMID 1715244. S2CID 22094672.
- Mandanas RA. “Graft Versus Host Disease Treatment & Management: Medical Care”. Medscape. Retrieved 30 August 2017.
Other substances that have been studied for GvHD treatment include, for example: sirolimus, pentostatin, etanercept, and alemtuzumab.
- Mandanas RA. “Graft Versus Host Disease Treatment & Management: Medical Care”. Medscape. Retrieved 30 August 2017.
In August 2017, the US FDA approved ibrutinib to treat chronic GvHD after failure of one or more other systemic treatments.
- Research, Center for Drug Evaluation and (February 9, 2019). “FDA expands ibrutinib indications to chronic GVHD”. FDA – via www.fda.gov.
Clinical research
There are a large number of clinical trials either ongoing or recently completed in the investigation of graft-versus-host disease treatment and prevention.
- “Search of: Graft-versus-host disease – List Results – ClinicalTrials.gov”. clinicaltrials.gov.
On May 17, 2012, Osiris Therapeutics announced that Canadian health regulators approved Prochymal, its drug for acute graft-versus-host disease in children who have failed to respond to steroid treatment. Prochymal is the first stem cell drug to be approved for a systemic disease.
- “World’s First Stem-Cell Drug Approval Achieved in Canada”. The National Law Review. Drinker Biddle & Reath LLP. 2012-06-12. Retrieved 2012-07-01.
In January 2016, Mesoblast released results of a phase 2 clinical trial on 241 children with acute Graft-versus-host disease, that was not responsive to steroids. The trial was of a mesenchymal stem cell therapy known as remestemcel-L or MSC-100-IV. Survival rate was 82% (vs 39% of controls) for those who showed some improvement after one month, and in the long term 72% (vs 18% of controls) for those that showed little effect after one month.
- “Increased Survival Using MSB Cells In Children With aGVHD”. Retrieved 22 Feb 2016.
HIV elimination
Graft-versus-host disease has been implicated in eliminating several cases of HIV, including The Berlin Patient and six others in Spain.
- “Immune war with donor cells after transplant may wipe out HIV”. ?. NewScientist. 2017-05-03. Retrieved 2018-11-23.
See also
References
- Williamson, Lorna M. (1998-09-01). “Transfusion associated graft versus host disease and its prevention”. Heart. 80 (3): 211–212. doi:10.1136/hrt.80.3.211. ISSN 1355-6037. PMC 1761088. PMID 9875072.
- Ghimire S, Weber D, Mavin E, Wang XN, Dickinson AM, Holler E (2017). “Pathophysiology of GvHD and Other HSCT-Related Major Complications”. Frontiers in Immunology. 8: 79. doi:10.3389/fimmu.2017.00079. PMC 5357769. PMID 28373870.
- Martino R, Romero P, Subirá M, Bellido M, Altés A, Sureda A, et al. (August 1999). “Comparison of the classic Glucksberg criteria and the IBMTR Severity Index for grading acute graft-versus-host disease following HLA-identical sibling stem cell transplantation. International Bone Marrow Transplant Registry”. Bone Marrow Transplantation. 24 (3): 283–7. doi:10.1038/sj.bmt.1701899. PMID 10455367. S2CID 24811357.
- Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. (December 2005). “National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report”. Biology of Blood and Marrow Transplantation. 11 (12): 945–56. doi:10.1016/j.bbmt.2005.09.004. PMC 4329079. PMID 16338616.
- Morisse-Pradier H, Nove-Josserand R, Philit F, Senechal A, Berger F, Callet-Bauchu E, et al. (February 2016). “[Graft-versus-host disease, a rare complication of lung transplantation]”. Revue de Pneumologie Clinique. 72 (1): 101–7. doi:10.1016/j.pneumo.2015.05.004. PMID 26209034.
- Paczesny S, Braun TM, Levine JE, Hogan J, Crawford J, Coffing B, et al. (January 2010). “Elafin is a biomarker of graft-versus-host disease of the skin”. Science Translational Medicine. 2 (13): 13–14. doi:10.1016/j.bbmt.2008.12.039. PMC 2895410. PMID 20371463.
- Ogawa Y, Shimmura S, Dogru M, Tsubota K (November 2010). “Immune processes and pathogenic fibrosis in ocular chronic graft-versus-host disease and clinical manifestations after allogeneic hematopoietic stem cell transplantation”. Cornea. 29 Suppl 1 (Nov Supplement 1): S68-77. doi:10.1097/ICO.0b013e3181ea9a6b. PMID 20935546. S2CID 39209313.
- Spiryda LB, Laufer MR, Soiffer RJ, Antin JA (December 2003). “Graft-versus-host disease of the vulva and/or vagina: diagnosis and treatment”. Biology of Blood and Marrow Transplantation. 9 (12): 760–5. doi:10.1016/j.bbmt.2003.08.001. PMID 14677115.
- Funke VA, Moreira MC, Vigorito AC (October 2016). “Acute and chronic Graft-versus-host disease after hematopoietic stem cell transplantation”. Revista da Associação Médica Brasileira. 62 (supl 1): 44–50. doi:10.1590/1806-9282.62.suppl1.44. PMID 27982319.
- “Stem Cell or Bone Marrow Transplant Side Effects”. www.cancer.org. Retrieved 2020-09-01.
- Goker H, Haznedaroglu IC, Chao NJ (March 2001). “Acute graft-vs-host disease: pathobiology and management”. Experimental Hematology. 29 (3): 259–77. doi:10.1016/S0301-472X(00)00677-9. PMID 11274753.
- “Graft-versus-host disease”. MedlinePlus. National Library of Medicine. Retrieved 6 May 2019.
- Krejci M, Kamelander J, Pospisil Z, Mayer J (2012). “Kinetics of bilirubin and liver enzymes is useful for predicting of liver graft-versus-host disease”. Neoplasma. 59 (3): 264–8. doi:10.4149/neo_2012_034. PMID 22296496.
- Feito-Rodríguez M, de Lucas-Laguna R, Gómez-Fernández C, Sendagorta-Cudós E, Collantes E, Beato MJ, Boluda ER (2013). “Cutaneous graft versus host disease in pediatric multivisceral transplantation”. Pediatric Dermatology. 30 (3): 335–41. doi:10.1111/j.1525-1470.2012.01839.x. PMID 22957989. S2CID 25151282.
- El-Jawahri A, Li S, Antin JH, Spitzer TR, Armand PA, Koreth J, et al. (May 2016). “Improved Treatment-Related Mortality and Overall Survival of Patients with Grade IV Acute GVHD in the Modern Years”. Biology of Blood and Marrow Transplantation. 22 (5): 910–8. doi:10.1016/j.bbmt.2015.12.024. PMID 26748160.
- Lee SJ, Vogelsang G, Flowers ME (April 2003). “Chronic graft-versus-host disease”. Biology of Blood and Marrow Transplantation. 9 (4): 215–33. doi:10.1053/bbmt.2003.50026. PMID 12720215.
- Tsukada S, Itonaga H, Taguchi J, Miyoshi T, Hayashida S, Sato S, et al. (2019). “[Gingival squamous cell carcinoma diagnosed on the occasion of osteonecrosis of the jaw in a patient with chronic GVHD]”. [Rinsho Ketsueki] the Japanese Journal of Clinical Hematology. 60 (1): 22–27. doi:10.11406/rinketsu.60.22. PMID 30726819.
- Billingham RE (1966). “The biology of graft-versus-host reactions”. Harvey Lectures. 62 (62): 21–78. PMID 4875305.
- Yasuda H, Ohto H, Abe R (1993). “Mechanism of transfusion-associated graft-versus-host disease”. Fukushima J Med Sci. 39 (2): 69–75. PMID 7927137.
- Kanda J (September 2013). “Effect of HLA mismatch on acute graft-versus-host disease”. International Journal of Hematology. 98 (3): 300–8. doi:10.1007/s12185-013-1405-x. PMID 23893313. S2CID 1585777.
- Bonifazi F, Solano C, Wolschke C, Sessa M, Patriarca F, Zallio F, et al. (February 2019). “Acute GVHD prophylaxis plus ATLG after myeloablative allogeneic haemopoietic peripheral blood stem-cell transplantation from HLA-identical siblings in patients with acute myeloid leukaemia in remission: final results of quality of life and long-term outcome analysis of a phase 3 randomised study”. The Lancet. Haematology. 6 (2): e89–e99. doi:10.1016/S2352-3026(18)30214-X. hdl:10138/311714. PMID 30709437. S2CID 73449161.
- Taylor CJ, Bolton EM, Bradley JA (August 2011). “Immunological considerations for embryonic and induced pluripotent stem cell banking”. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 366 (1575): 2312–22. doi:10.1098/rstb.2011.0030. PMC 3130422. PMID 21727137.
- Solomon S, Pitossi F, Rao MS (February 2015). “Banking on iPSC–is it doable and is it worthwhile”. Stem Cell Reviews and Reports. 11 (1): 1–10. doi:10.1007/s12015-014-9574-4. PMC 4333229. PMID 25516409.
- Falkenburg JH, Jedema I (December 2017). “Graft versus tumor effects and why people relapse”. Hematology. American Society of Hematology. Education Program. 2017 (1): 693–698. doi:10.1182/asheducation-2017.1.693. PMC 6142614. PMID 29222323.
- Sun K, Li M, Sayers TJ, Welniak LA, Murphy WJ (August 2008). “Differential effects of donor T-cell cytokines on outcome with continuous bortezomib administration after allogeneic bone marrow transplantation”. Blood. 112 (4): 1522–9. doi:10.1182/blood-2008-03-143461. PMC 2515132. PMID 18539902.
- Moroff G, Leitman SF, Luban NL (October 1997). “Principles of blood irradiation, dose validation, and quality control”. Transfusion. 37 (10): 1084–92. doi:10.1046/j.1537-2995.1997.371098016450.x. PMID 9354830. S2CID 7462268.
- Xia G, Goebels J, Rutgeerts O, Vandeputte M, Waer M (February 2001). “Transplantation tolerance and autoimmunity after xenogeneic thymus transplantation”. Journal of Immunology. 166 (3): 1843–54. doi:10.4049/jimmunol.166.3.1843. PMID 11160231. S2CID 24007739.
- Markert ML, Devlin BH, McCarthy EA, Chinn IK, Hale LP (2008). “Thymus Transplantation”. In Lavini C, Moran CA, Morandi U, et al. (eds.). Thymus Gland Pathology: Clinical, Diagnostic, and Therapeutic Features. pp. 255–267. doi:10.1007/978-88-470-0828-1_30. ISBN 978-88-470-0827-4. S2CID 219575147.
- Markert ML, Devlin BH, Alexieff MJ, Li J, McCarthy EA, Gupton SE, et al. (May 2007). “Review of 54 patients with complete DiGeorge anomaly enrolled in protocols for thymus transplantation: outcome of 44 consecutive transplants”. Blood. 109 (10): 4539–47. doi:10.1182/blood-2006-10-048652. PMC 1885498. PMID 17284531.
- Wadhera A, Maverakis E, Mitsiades N, Lara PN, Fung MA, Lynch PJ (October 2007). “Thymoma-associated multiorgan autoimmunity: a graft-versus-host-like disease”. Journal of the American Academy of Dermatology. 57 (4): 683–9. doi:10.1016/j.jaad.2007.02.027. PMID 17433850.
- Nassereddine S, Rafei H, Elbahesh E, Tabbara I (April 2017). “Versus Host Disease: A Comprehensive Review”. Anticancer Research. 37 (4): 1547–1555. doi:10.21873/anticanres.11483. PMID 28373413.
- Roncarolo MG, Battaglia M (August 2007). “Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans”. Nature Reviews. Immunology. 7 (8): 585–98. doi:10.1038/nri2138. PMID 17653126. S2CID 7043844.
- Zhang L, Chu J, Yu J, Wei W (February 2016). “Cellular and molecular mechanisms in graft-versus-host disease”. Journal of Leukocyte Biology. 99 (2): 279–87. doi:10.1189/jlb.4ru0615-254rr. PMID 26643713. S2CID 25250676.
- Morishima Y, Sasazuki T, Inoko H, Juji T, Akaza T, Yamamoto K, et al. (June 2002). “The clinical significance of human leukocyte antigen (HLA) allele compatibility in patients receiving a marrow transplant from serologically HLA-A, HLA-B, and HLA-DR matched unrelated donors”. Blood. 99 (11): 4200–6. doi:10.1182/blood.V99.11.4200. PMID 12010826. S2CID 6859250.
- Grewal SS, Barker JN, Davies SM, Wagner JE (June 2003). “Unrelated donor hematopoietic cell transplantation: marrow or umbilical cord blood?”. Blood. 101 (11): 4233–44. doi:10.1182/blood-2002-08-2510. PMID 12522002. S2CID 6486524.
- Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE, et al. (June 2001). “Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors”. The New England Journal of Medicine. 344 (24): 1815–22. doi:10.1056/NEJM200106143442402. PMID 11407342.
- Törlén J, Ringdén O, Garming-Legert K, Ljungman P, Winiarski J, Remes K, et al. (November 2016). “A prospective randomized trial comparing cyclosporine/methotrexate and tacrolimus/sirolimus as graft-versus-host disease prophylaxis after allogeneic hematopoietic stem cell transplantation”. Haematologica. 101 (11): 1417–1425. doi:10.3324/haematol.2016.149294. PMC 5394879. PMID 27662016.
- Fisher SA, Cutler A, Doree C, Brunskill SJ, Stanworth SJ, Navarrete C, Girdlestone J, et al. (Cochrane Haematological Malignancies Group) (January 2019). “Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition”. The Cochrane Database of Systematic Reviews. 1 (1): CD009768. doi:10.1002/14651858.CD009768.pub2. PMC 6353308. PMID 30697701.
- Hale G, Waldmann H (May 1994). “Control of graft-versus-host disease and graft rejection by T cell depletion of donor and recipient with Campath-1 antibodies. Results of matched sibling transplants for malignant diseases”. Bone Marrow Transplantation. 13 (5): 597–611. PMID 8054913.
- Wagner JE, Thompson JS, Carter SL, Kernan NA (2005). “Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II-III trial”. Lancet. 366 (9487): 733–41. doi:10.1016/S0140-6736(05)66996-6. PMID 16125590. S2CID 33732870.
- Menillo SA, Goldberg SL, McKiernan P, Pecora AL (October 2001). “Intraoral psoralen ultraviolet A irradiation (PUVA) treatment of refractory oral chronic graft-versus-host disease following allogeneic stem cell transplantation”. Bone Marrow Transplantation. 28 (8): 807–8. doi:10.1038/sj.bmt.1703231. PMID 11781637. S2CID 27292769.
- Flinn, Aisling M; Gennery, Andrew R (2023-03-06). “Recent advances in graft-versus-host disease”. Faculty Reviews. 12: 4. doi:10.12703/r/12-4. ISSN 2732-432X. PMC 10009889. PMID 36923700.
- Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL (August 1991). “Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes”. Cell. 66 (4): 807–15. doi:10.1016/0092-8674(91)90124-h. PMID 1715244. S2CID 22094672.
- Mandanas RA. “Graft Versus Host Disease Treatment & Management: Medical Care”. Medscape. Retrieved 30 August 2017.
- Research, Center for Drug Evaluation and (February 9, 2019). “FDA expands ibrutinib indications to chronic GVHD”. FDA – via www.fda.gov.
- “Search of: Graft-versus-host disease – List Results – ClinicalTrials.gov”. clinicaltrials.gov.
- “World’s First Stem-Cell Drug Approval Achieved in Canada”. The National Law Review. Drinker Biddle & Reath LLP. 2012-06-12. Retrieved 2012-07-01.
- “Increased Survival Using MSB Cells In Children With aGVHD”. Retrieved 22 Feb 2016.
- “Immune war with donor cells after transplant may wipe out HIV”. ?. NewScientist. 2017-05-03. Retrieved 2018-11-23.
- GRCh38: Ensembl release 89: ENSG00000124102 – Ensembl, May 2017
- “Human PubMed Reference:”. National Center for Biotechnology Information, U.S. National Library of Medicine.
- Molhuizen HO, Zeeuwen PL, Olde Weghuis D, Geurts van Kessel A, Schalkwijk J (Feb 1994). “Assignment of the human gene encoding the epidermal serine proteinase inhibitor SKALP (PI3) to chromosome region 20q12→q13”. Cytogenet Cell Genet. 66 (2): 129–31. doi:10.1159/000133683. PMID 8287685.
- Clauss A, Lilja H, Lundwall A (Nov 2002). “A locus on human chromosome 20 contains several genes expressing protease inhibitor domains with homology to whey acidic protein”. Biochem J. 368 (Pt 1): 233–42. doi:10.1042/BJ20020869. PMC 1222987. PMID 12206714.
- “Entrez Gene: PI3 peptidase inhibitor 3, skin-derived (SKALP)”.
- Paczesny S, Levine JE, Hogan J, Crawford J, Braun TM, Wang H, Faca V, Zhang Q, Pitteri S, Chin A, Choi SW, Kitko CL, Krijanovski OI, Reddy P, Mineishi S, Whitfield J, Jones S, Hanash SM, Ferrara JLM (February 2009). “[Elafin is a Biomarker of Graft Versus Host Disease of the Skin”. Biology of Blood and Marrow Transplantation. 15 (2 Suppl 1): 13–14. doi:10.1016/j.bbmt.2008.12.039. PMC 2895410. PMID 20371463.
- “Archives”. Los Angeles Times. November 2012.
- “Complications of Transfusion: Transfusion Medicine: Merck Manual Professional”. Retrieved 2009-02-09.
- Savage WJ (June 2016). “Transfusion Reactions”. Hematology/Oncology Clinics of North America. 30 (3): 619–634. doi:10.1016/j.hoc.2016.01.012. PMID 27113000.
- Vaillant AA, Modi P, Mohammadi O (2022). “Graft Versus Host Disease”. StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 30855823. Retrieved 2023-02-02.
- “National Healthcare Safety Network (NHSN)”. www.cdc.gov. 2017-12-29. Retrieved 2018-09-18.
- Patel KK, Patel AK, Ranjan RR, Shah AP (September 2010). “Transfusion associated graft versus host disease following whole blood transfusion from an unrelated donor in an immunocompetent patient”. Indian Journal of Hematology & Blood Transfusion. 26 (3): 92–95. doi:10.1007/s12288-010-0028-0. PMC 3002081. PMID 21886390.
- A Case of Fetal-Induced Graft-versus-Host Disease
- Fung MK, Grossman BJ, Hillyer CD, Westhoff CM (2014). Technical manual (18th ed.). Bethesda, Md.: American Association of Blood Banks. ISBN 978-1563958885. OCLC 881812415.
- Wadhera A, Maverakis E, Mitsiades N, Lara PN, Fung MA, Lynch PJ (Oct 2007). “Thymoma-associated multiorgan autoimmunity: a graft-versus-host-like disease”. J Am Acad Dermatol. 57 (4): 683–9. doi:10.1016/j.jaad.2007.02.027. PMID 17433850.
- Meneshian A, Giaccone G, Olivier KR. “Clinical presentation and management of thymoma and thymic carcinoma”. UpToDate.
- Nakayama M, Itoh M, Kikuchi S, Tanito K, Nakagawa H (Apr 2016). “Thymoma-associated cutaneous graft-versus-host-like disease possibly treated with Narrow-band UVB phototherapy”. Eur J Dermatol. 26 (2): 208–9. doi:10.1684/ejd.2015.2716. PMID 27018042. S2CID 26959211.
- Kornacki S, Hansen FC, Lazenby A (1995). “Graft-versus-host-like colitis associated with malignant thymoma”. Am J Surg Pathol. 19 (2): 224–8. doi:10.1097/00000478-199502000-00011. PMID 7832281.
Further reading
- Ferrara JLM, Deeg HJ, Burakoff SJ. Graft-Vs.-Host Disease: Immunology, Pathophysiology, and Treatment. Marcel Dekker, 1990 ISBN 0-8247-9728-0
- Polsdorfer, JR Gale Encyclopedia of Medicine: Graft-vs.-host disease
- Anwar M, Bhatti FA (2003). “Transfusion associated graft versus host disease”. Journal of Ayub Medical College, Abbottabad. 15 (3): 56–58. PMID 14727344.
- Gupta A, Bansal D, Dass R, Das A (December 2004). “Transfusion associated graft versus host disease” (PDF). Indian Pediatrics. 41 (12): 1260–1264. PMID 15623910.
- Triulzi DJ (September 1992). “Transfusion associated graft vs. host disease and irradiated blood components”. Archived from the original on 2006-07-22.
- Kardon E (8 July 2022). “Transfusion Reactions”. EMedicine.
- Sallenave JM (2003). “The role of secretory leukocyte proteinase inhibitor and elafin (elastase-specific inhibitor/skin-derived antileukoprotease) as alarm antiproteinases in inflammatory lung disease”. Respir. Res. 1 (2): 87–92. doi:10.1186/rr18. PMC 59548. PMID 11667971.
- Saheki T, Ito F, Hagiwara H, et al. (1992). “Primary structure of the human elafin precursor preproelafin deduced from the nucleotide sequence of its gene and the presence of unique repetitive sequences in the prosegment”. Biochem. Biophys. Res. Commun. 185 (1): 240–5. doi:10.1016/S0006-291X(05)80981-7. PMID 1339270.
- Sallenave JM, Marsden MD, Ryle AP (1992). “Isolation of elafin and elastase-specific inhibitor (ESI) from bronchial secretions. Evidence of sequence homology and immunological cross-reactivity”. Biol. Chem. Hoppe-Seyler. 373 (1): 27–33. doi:10.1515/bchm3.1992.373.1.27. PMID 1536690.
- Chang A, Schalkwijk J, Happle R, van de Kerkhof PC (1990). “Elastase-inhibiting activity in scaling skin disorders”. Acta Derm. Venereol. 70 (2): 147–51. doi:10.2340/0001555570147151. PMID 1969201.
- Schalkwijk J, de Roo C, de Jongh GJ (1991). “Skin-derived antileukoproteinase (SKALP), an elastase inhibitor from human keratinocytes. Purification and biochemical properties”. Biochim. Biophys. Acta. 1096 (2): 148–54. doi:10.1016/0925-4439(91)90053-c. PMID 2001428.
- Sallenave JM, Ryle AP (1991). “Purification and characterization of elastase-specific inhibitor. Sequence homology with mucus proteinase inhibitor”. Biol. Chem. Hoppe-Seyler. 372 (1): 13–21. doi:10.1515/bchm3.1991.372.1.13. PMID 2039600.
- Wiedow O, Schröder JM, Gregory H, et al. (1990). “Elafin: an elastase-specific inhibitor of human skin. Purification, characterization, and complete amino acid sequence”. J. Biol. Chem. 265 (25): 14791–5. doi:10.1016/S0021-9258(18)77182-2. PMID 2394696.
- Molhuizen HO, Alkemade HA, Zeeuwen PL, et al. (1993). “SKALP/elafin: an elastase inhibitor from cultured human keratinocytes. Purification, cDNA sequence, and evidence for transglutaminase cross-linking”. J. Biol. Chem. 268 (16): 12028–32. doi:10.1016/S0021-9258(19)50303-9. PMID 7685029.
- Zhang M, Zou Z, Maass N, Sager R (1995). “Differential expression of elafin in human normal mammary epithelial cells and carcinomas is regulated at the transcriptional level”. Cancer Res. 55 (12): 2537–41. PMID 7780965.
- Sallenave JM, Silva A (1993). “Characterization and gene sequence of the precursor of elafin, an elastase-specific inhibitor in bronchial secretions”. Am. J. Respir. Cell Mol. Biol. 8 (4): 439–45. doi:10.1165/ajrcmb/8.4.439. PMID 8476637.
- Tsunemi M, Matsuura Y, Sakakibara S, Katsube Y (1996). “Crystal structure of an elastase-specific inhibitor elafin complexed with porcine pancreatic elastase determined at 1.9 A resolution”. Biochemistry. 35 (36): 11570–6. doi:10.1021/bi960900l. PMID 8794736.
- Pfundt R, van Ruissen F, van Vlijmen-Willems IM, et al. (1996). “Constitutive and inducible expression of SKALP/elafin provides anti-elastase defense in human epithelia”. J. Clin. Invest. 98 (6): 1389–99. doi:10.1172/JCI118926. PMC 507565. PMID 8823304.
- Steinert PM, Marekov LN (1997). “Direct evidence that involucrin is a major early isopeptide cross-linked component of the keratinocyte cornified cell envelope”. J. Biol. Chem. 272 (3): 2021–30. doi:10.1074/jbc.272.3.2021. PMID 8999895.
- Francart C, Dauchez M, Alix AJ, Lippens G (1997). “Solution structure of R-elafin, a specific inhibitor of elastase”. J. Mol. Biol. 268 (3): 666–77. doi:10.1006/jmbi.1997.0983. PMID 9171290.
- Kuijpers AL, Pfundt R, Zeeuwen PL, et al. (1998). “SKALP/elafin gene polymorphisms are not associated with pustular forms of psoriasis”. Clin. Genet. 54 (1): 96–101. doi:10.1111/j.1399-0004.1998.tb03703.x. PMID 9727750. S2CID 34775360.
- Mihaila A, Tremblay GM (2001). “Human alveolar macrophages express elafin and secretory leukocyte protease inhibitor”. Z. Naturforsch. C. 56 (3–4): 291–7. doi:10.1515/znc-2001-3-420. PMID 11371023. S2CID 29205936.
- Simpson AJ, Wallace WA, Marsden ME, et al. (2001). “Adenoviral augmentation of elafin protects the lung against acute injury mediated by activated neutrophils and bacterial infection”. J. Immunol. 167 (3): 1778–86. doi:10.4049/jimmunol.167.3.1778. PMID 11466403.
- Sumi Y, Inoue N, Azumi H, et al. (2002). “Expression of tissue transglutaminase and elafin in human coronary artery: implication for plaque instability”. Atherosclerosis. 160 (1): 31–9. doi:10.1016/S0021-9150(01)00542-1. PMID 11755920.
External links
| Classification | DICD–10: T86.0ICD–9-CM: 279.50MeSH: D006086DiseasesDB: 5388 |
|---|---|
| External resources | MedlinePlus: 001309eMedicine: med/926 ped/893 derm/478 |
| Consequences of external causes |
|---|


Leave a Reply