Microsomal prostaglandin E synthase-1 (mPGES-1) or Prostaglandin E synthase is an enzyme that in humans is encoded by the PTGES gene.
- Jakobsson PJ, Thorén S, Morgenstern R, et al. (1989). “Identification of human prostaglandin E synthase: a microsomal glutathione-dependent, inducible enzyme, constituting a potential novel drug target”. Proc Natl Acad Sci USA. 96 (13): 7220–7225. Bibcode:1999PNAS…96.7220J. doi:10.1073/pnas.96.13.7220. PMC 22058. PMID 10377395.
- Hui-Hua Chang; Emmanuelle J Meuillet (2011). “Identification and development of mPGES-1 inhibitors: where we are at?”. Future Med Chem. 3 (15): 1909–1934. doi:10.4155/fmc.11.136. PMC 3232027. PMID 22023034.
- Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B (Sep 1997). “A model for p53-induced apoptosis”. Nature. 389 (6648): 300–5. Bibcode:1997Natur.389..300P. doi:10.1038/38525. PMID 9305847. S2CID 4429638.
- Jakobsson PJ, Morgenstern R, Mancini J, Ford-Hutchinson A, Persson B (May 1999). “Common structural features of MAPEG — a widespread superfamily of membrane-associated proteins with highly divergent functions in eicosanoid and glutathione metabolism”. Protein Sci. 8 (3): 689–92. doi:10.1110/ps.8.3.689. PMC 2144274. PMID 10091672.
- “Entrez Gene: PTGES prostaglandin E synthase”.
The protein encoded by this gene is a glutathione-dependent prostaglandin E synthase. The expression of this gene has been shown to be induced by proinflammatory cytokine interleukin 1 beta (IL1B). Its expression can also be induced by tumor suppressor protein TP53, and may be involved in TP53-induced apoptosis.
Knockout studies in mice suggest that this gene may contribute to the pathogenesis of collagen-induced arthritis and mediate acute pain during inflammatory responses.
Gold sodium thiomalate
Sodium aurothiomalate (INN, known in the United States as gold sodium thiomalate) is a gold compound that is used for its immunosuppressive anti-rheumatic effects. Along with an orally-administered gold salt, auranofin, it is one of only two gold compounds currently employed in modern medicine. Its precise mechanism of action is unknown but is known that it inhibits the synthesis of prostaglandins. It also modulates phagocytic cells and inhibits class II major histocompatibility complex-peptide interactions. It is also known that it inhibits the following enzymes:
- Acid phosphatase
- Beta-glucuronidase
- Elastase
- Cathepsin G
- Thrombin
- Microsomal prostaglandin E synthase-1
- Jessop JD, O’Sullivan MM, Lewis PA, Williams LA, Camilleri JP, Plant MJ, Coles EC (September 1998). “A long-term five-year randomized controlled trial of hydroxychloroquine, sodium aurothiomalate, auranofin and penicillamine in the treatment of patients with rheumatoid arthritis”. British Journal of Rheumatology. 37 (9): 992–1002. doi:10.1093/rheumatology/37.9.992. PMID 9783766.
- Iqbal MS, Saeed M, Taqi SG (2008). “Erythrocyte membrane gold levels after treatment with auranofin and sodium aurothiomalate”. Biological Trace Element Research. 126 (1–3): 56–64. doi:10.1007/s12011-008-8184-x. PMID 18649049. S2CID 20169992.
- Kean WF, Kean IR (June 2008). “Clinical pharmacology of gold”. Inflammopharmacology. 16 (3): 112–25. doi:10.1007/s10787-007-0021-x. PMID 18523733. S2CID 808858.
- Berners-Price SJ, Filipovska A (September 2011). “Gold compounds as therapeutic agents for human diseases”. Metallomics. 3 (9): 863–73. doi:10.1039/c1mt00062d. PMID 21755088.
- Tuure L, Hämäläinen M, Moilanen T, Moilanen E (2014). “Aurothiomalate inhibits the expression of mPGES-1 in primary human chondrocytes”. Scandinavian Journal of Rheumatology. 44 (1): 74–9. doi:10.3109/03009742.2014.927917. PMID 25314295. S2CID 5213201.
See also
Prostaglandin E synthase (EC 5.3.99.3, or PGE synthase) is an enzyme involved in eicosanoid and glutathione metabolism, a member of MAPEG family. It generates prostaglandin E (PGE) from prostaglandin H2.
- Murakami M, Nakatani Y, Tanioka T, Kudo I (August 2002). “Prostaglandin E synthase”. Prostaglandins Other Lipid Mediat. 68–69: 383–99. doi:10.1016/S0090-6980(02)00043-6. PMID 12432931. S2CID 22246484.
- Park JY, Pillinger MH, Abramson SB (June 2006). “Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases”. Clin. Immunol. 119 (3): 229–40. doi:10.1016/j.clim.2006.01.016. PMID 16540375.
Prostaglandin E is a family of naturally occurring prostaglandins that are used as medications.
Types include:
- Prostaglandin E1 also known as alprostadil
- Prostaglandin E1 (PGE1) is a naturally occurring prostaglandin and is also used as a medication (alprostadil).In infants with congenital heart defects, it is delivered by slow injection into a vein to open the ductus arteriosus until surgery can be carried out. By injection into the penis or placement in the urethra, it is used to treat erectile dysfunction. Common side effects when given to babies include decreased breathing, fever, and low blood pressure. When injected into the penis for erectile dysfunction; side effects may include penile pain, bleeding at the site of injection, and prolonged erection (priapism). Prostaglandin E1 is in the vasodilator family of medications. It works by opening blood vessels and relaxing smooth muscle. Prostaglandin E1 was isolated in 1957 and approved for medical use in the United States in 1981. It is on the World Health Organization’s List of Essential Medicines.
- Misoprostol is another synthetic prostaglandin E1 analog used to prevent gastric ulcers when taken on a continuous basis, to treat missed miscarriage, to induce labor, and to induce abortion.
- “FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)”. nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- “Alprostadil”. The American Society of Health-System Pharmacists. Archived from the original on 16 January 2017. Retrieved 8 January 2017.
- Northern Neonatal Network (208). Neonatal Formulary: Drug Use in Pregnancy and the First Year of Life (5 ed.). John Wiley & Sons. p. 2010. ISBN 9780470750353. Archived from the original on 13 January 2017.
- British National Formulary (BNF) (69th ed.). British Medical Association. 2015. p. 569. ISBN 9780857111562.
- Sneader W (2005). Drug Discovery: A History. John Wiley & Sons. p. 185. ISBN 9780470015520. Archived from the original on 13 January 2017.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- Wu HL, Marwah S, Wang P, Wang QM, Chen XW (May 2017). “Misoprostol for medical treatment of missed abortion: a systematic review and network meta-analysis”. Scientific Reports. 7 (1): 1664. Bibcode:2017NatSR…7.1664W. doi:10.1038/s41598-017-01892-0. PMC 5431938. PMID 28490770.
- Chatsis V, Frey N (2018). Misoprostol for Cervical Ripening and Induction of Labour: A Review of Clinical Effectiveness, Cost-Effectiveness and Guidelines. CADTH Rapid Response Reports. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health. PMID 30907996.
- “Medical abortion”. Mayo Clinic. Retrieved 28 April 2022.
- Prostaglandin E2 also known as dinoprostone
- Prostaglandin E2 (PGE2), also known as dinoprostone, is a naturally occurring prostaglandin with oxytocic properties that is used as a medication. Dinoprostone is used in labor induction, bleeding after delivery, termination of pregnancy, and in newborn babies to keep the ductus arteriosus open. In babies it is used in those with congenital heart defects until surgery can be carried out. It is also used to manage gestational trophoblastic disease. It may be used within the vagina or by injection into a vein. Dinoprostone has important effects in labor by inducing softening of the cervix and causing uterine contraction, and also stimulates osteoblasts to release factors that stimulate bone resorption by osteoclasts. Natural prostaglandins, including PGE1 and PGE2, are important in the structure and function of the ductus arteriosus in fetuses and newborns. They allow the ductus arteriosus to remain open, providing the necessary connection between the pulmonary artery and descending aorta that allows the blood to bypass the fetus’s underdeveloped lungs and be transported to the placenta for oxygenation. Prostaglandin E2 was first synthesized in 1970 and approved for medical use by the FDA in the United States in 1977. It is on the World Health Organization’s List of Essential Medicines. Prostaglandin E2 works as well as prostaglandin E1 in babies.
- “Dinoprostone topical Use During Pregnancy”. Drugs.com. 17 December 2019. Retrieved 27 July 2020.
- “Dinoprostone”. The American Society of Health-System Pharmacists. Archived from the original on 16 January 2017. Retrieved 8 January 2017.
- Shirley M (October 2018). “Dinoprostone Vaginal Insert: A Review in Cervical Ripening”. Drugs. 78 (15): 1615–1624. doi:10.1007/s40265-018-0995-2. PMID 30317521. S2CID 52978808.
- Xi M, Gerriets V (2020). “Prostaglandin E2 (Dinoprostone)”. StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 31424863.
- Northern Neonatal Network (208). Neonatal Formulary: Drug Use in Pregnancy and the First Year of Life (5 ed.). John Wiley & Sons. p. 2010. ISBN 9780470750353. Archived from the original on 2017-01-13.
- British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. pp. 538–540. ISBN 9780857111562.
- Hwa J, Martin K (2017). “Chapter 18: The Eicosanoids: Prostaglandins, Thromboxanes, Leukotrienes, & Related Compounds”. In Katzung BG (ed.). Basic & Clinical Pharmacology (14th ed.). New York, NY: McGraw-Hill Education.
- Smith WL, Urade Y, Jakobsson PJ (October 2011). “Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis”. Chemical Reviews. 111 (10): 5821–65. doi:10.1021/cr2002992. PMC 3285496. PMID 21942677.
- Hamilton R (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 361. ISBN 9781284057560.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- “Dinoprostone”. PubChem. Retrieved 2020-07-27.
- Sharma M, Sasikumar M, Karloopia SD, Shahi BN (April 2001). “Prostaglandins in Congential Heart Disease”. Medical Journal, Armed Forces India. 57 (2): 134–8. doi:10.1016/S0377-1237(01)80134-9. PMC 4925861. PMID 27407318.
- Prostaglandin E2 (PGE2), also known as dinoprostone, is a naturally occurring prostaglandin with oxytocic properties that is used as a medication. Dinoprostone is used in labor induction, bleeding after delivery, termination of pregnancy, and in newborn babies to keep the ductus arteriosus open. In babies it is used in those with congenital heart defects until surgery can be carried out. It is also used to manage gestational trophoblastic disease. It may be used within the vagina or by injection into a vein. Dinoprostone has important effects in labor by inducing softening of the cervix and causing uterine contraction, and also stimulates osteoblasts to release factors that stimulate bone resorption by osteoclasts. Natural prostaglandins, including PGE1 and PGE2, are important in the structure and function of the ductus arteriosus in fetuses and newborns. They allow the ductus arteriosus to remain open, providing the necessary connection between the pulmonary artery and descending aorta that allows the blood to bypass the fetus’s underdeveloped lungs and be transported to the placenta for oxygenation. Prostaglandin E2 was first synthesized in 1970 and approved for medical use by the FDA in the United States in 1977. It is on the World Health Organization’s List of Essential Medicines. Prostaglandin E2 works as well as prostaglandin E1 in babies.
Both types are on the World Health Organization’s List of Essential Medicines. Prostaglandin E play an important role in thermoregulation of the human brain. Decreased formation of prostaglandin E through inhibition of cyclooxygenase is the basis for the antipyretic of nonsteroidal anti-inflammatory drugs (NSAIDs).
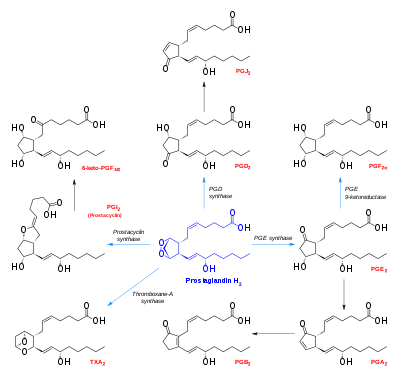
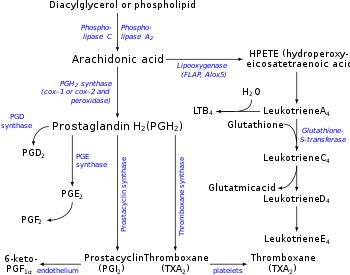
Prostaglandin H2 is a type of prostaglandin and a precursor for many other biologically significant molecules. It is synthesized from arachidonic acid in a reaction catalyzed by a cyclooxygenase enzyme. The conversion from Arachidonic acid to Prostaglandin H2 is a two step process. First, COX-1 catalyzes the addition of two free oxygens to form the 1,2-Dioxane bridge and a peroxide functional group to form Prostaglandin G2. Second, COX-2 reduces the peroxide functional group to a Secondary alcohol, forming Prostaglandin H2. Other peroxidases like Hydroquinone have been observed to reduce PGG2 to PGH2. PGH2 is unstable at room temperature, with a half life of 90-100 seconds, so it is often converted into a different prostaglandin.
- Wishart, David S.; Guo, An Chi; Oler, Eponine; Wang, Fel; Anjum, Afia; Peters, Harrison; Dizon, Raynard; Sayeeda, Zinat; Tian, Siyang; Lee, Brian L.; Berjanskii, Mark; Mah, Robert; Yamamoto, Mai; Jovel Castillo, Juan; Torres Calzada, Claudia; Hiebert Giesbrecht, Mickel; Lui, Vicki W.; Varshavi, Dorna; Varshavi, Dorsa; Allen, Dana; Arndt, David; Khetarpal, Nitya; Sivakumaran, Aadhavya; Harford, Karxena; Sanford, Selena; Yee, Kristen; Cao, Xuan; Budinsky, Zachary; Liigand, Jaanus; Zhang, Lun; Zheng, Jiamin; Mandal, Rupasri; Karu, Naama; Dambrova, Maija; Schiöth, Helgi B.; Gautam, Vasuk. “Showing metabocard for Prostaglandin H2 (HMDB0001381)”. Human Metabolome Database, HMDB. 5.0.
- van der Donk WA, Tsai AL, Kulmacz RJ (December 2002). “The cyclooxygenase reaction mechanism”. Biochemistry. 41 (52): 15451–8. doi:10.1021/bi026938h. PMID 12501173.
- Salomon RG, Miller DB, Zagorski MG, Coughlin DJ (October 1984). “Prostaglandin endoperoxides. 14. Solvent-induced fragmentation of prostaglandin endoperoxides. New aldehyde products from PGH2 and a novel intramolecular 1,2-hydride shift during endoperoxide fragmentation in aqueous solution”. Journal of the American Chemical Society. 106 (20): 6049–6060. doi:10.1021/ja00332a049. ISSN 0002-7863.
- Hla T, Neilson K (August 1992). “Human cyclooxygenase-2 cDNA”. Proceedings of the National Academy of Sciences of the United States of America. 89 (16): 7384–8. Bibcode:1992PNAS…89.7384H. doi:10.1073/pnas.89.16.7384. PMC 49714. PMID 1380156.
It is acted upon by:
- Prostacyclin synthase to create prostacyclin
- Thromboxane-A synthase to create thromboxane A2 and 12-(S)-hydroxy-5Z,8E,10E-heptadecatrienoic acid (HHT) (see 12-Hydroxyheptadecatrienoic acid)
- Prostaglandin D2 synthase to create prostaglandin D2
- Prostaglandin E synthase to create prostaglandin E2
It rearranges non-enzymatically to:
- A mixture of 12-(S)-hydroxy-5Z,8E,10E-heptadecatrienoic acid (HHT) and 12-(S)-hydroxy-5Z,8Z,10E-heptadecatrienoic acid (see 12-Hydroxyheptadecatrienoic acid)
- 12-Hydroxyheptadecatrienoic acid (also termed 12-HHT, 12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid, or 12(S)-HHTrE) is a 17 carbon metabolite of the 20 carbon polyunsaturated fatty acid, arachidonic acid. It was discovered and structurally defined in 1973 by P. Wlodawer, Bengt I. Samuelsson, and M. Hamberg, as a product of arachidonic acid metabolism made by microsomes (i.e. endoplasmic reticulum) isolated from sheep seminal vesicle glands and by intact human platelets.
- Wlodawer, P; Samuelsson, B (1973). “On the organization and mechanism of prostaglandin synthetase”. The Journal of Biological Chemistry. 248 (16): 5673–8. doi:10.1016/S0021-9258(19)43558-8. PMID 4723909.
- Hamberg, M; Samuelsson, B (1974). “Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets”. Proceedings of the National Academy of Sciences of the United States of America. 71 (9): 3400–4. Bibcode:1974PNAS…71.3400H. doi:10.1073/pnas.71.9.3400. PMC 433780. PMID 4215079.
- 12-Hydroxyheptadecatrienoic acid (also termed 12-HHT, 12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid, or 12(S)-HHTrE) is a 17 carbon metabolite of the 20 carbon polyunsaturated fatty acid, arachidonic acid. It was discovered and structurally defined in 1973 by P. Wlodawer, Bengt I. Samuelsson, and M. Hamberg, as a product of arachidonic acid metabolism made by microsomes (i.e. endoplasmic reticulum) isolated from sheep seminal vesicle glands and by intact human platelets.
Use of prostaglandin H2:
- regulating the constriction and dilation of blood vessels
- stimulating platelet aggregation
- binds to Thromboxane receptor on platelets’ cell membranes to trigger platelet migration and adhesion to other platelets.
- Woodward DF, Jones RL, Narumiya S (September 2011). “International Union of Basic and Clinical Pharmacology. LXXXIII: classification of prostanoid receptors, updating 15 years of progress”. Pharmacological Reviews. 63 (3): 471–538. doi:10.1124/pr.110.003517. PMID 21752876.
- binds to Thromboxane receptor on platelets’ cell membranes to trigger platelet migration and adhesion to other platelets.
Effects of Aspirin on prostaglandin H2:
- Aspirin has been hypothesized to block the conversion of arachidonic acid to prostaglandin
The synthase generating PGE2 is a membrane-associated protein.
Isozymes
Humans express three prostaglandin-E synthase isozymes, each encoded by a separate gene:
- prostaglandin E synthase (microsomal)
- prostaglandin E synthase 2 (microsomal)
- prostaglandin E synthase 3 (cytosolic)
- Prostaglandin E synthase 3 (cytosolic) is an enzyme that in humans is encoded by the PTGES3 gene. The protein encoded by this gene is also known as p23 which functions as a chaperone which is required for proper functioning of the glucocorticoid and other steroid receptors.
- “Entrez Gene: PTGES3 Prostaglandin E synthase 3 (cytosolic)”.
- Freeman BC, Yamamoto KR (June 2002). “Disassembly of transcriptional regulatory complexes by molecular chaperones”. Science. 296 (5576): 2232–2235. doi:10.1126/science.1073051. PMID 12077419. S2CID 8844179.
- Prostaglandin E synthase 3 (cytosolic) is an enzyme that in humans is encoded by the PTGES3 gene. The protein encoded by this gene is also known as p23 which functions as a chaperone which is required for proper functioning of the glucocorticoid and other steroid receptors.
References
- GRCh38: Ensembl release 89: ENSG00000148344 – Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000050737 – Ensembl, May 2017
- “Human PubMed Reference:”. National Center for Biotechnology Information, U.S. National Library of Medicine.
- “Mouse PubMed Reference:”. National Center for Biotechnology Information, U.S. National Library of Medicine.
- Jakobsson PJ, Thorén S, Morgenstern R, et al. (1989). “Identification of human prostaglandin E synthase: a microsomal glutathione-dependent, inducible enzyme, constituting a potential novel drug target”. Proc Natl Acad Sci USA. 96 (13): 7220–7225. Bibcode:1999PNAS…96.7220J. doi:10.1073/pnas.96.13.7220. PMC 22058. PMID 10377395.
- Hui-Hua Chang; Emmanuelle J Meuillet (2011). “Identification and development of mPGES-1 inhibitors: where we are at?”. Future Med Chem. 3 (15): 1909–1934. doi:10.4155/fmc.11.136. PMC 3232027. PMID 22023034.
- Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B (Sep 1997). “A model for p53-induced apoptosis”. Nature. 389 (6648): 300–5. Bibcode:1997Natur.389..300P. doi:10.1038/38525. PMID 9305847. S2CID 4429638.
- Jakobsson PJ, Morgenstern R, Mancini J, Ford-Hutchinson A, Persson B (May 1999). “Common structural features of MAPEG — a widespread superfamily of membrane-associated proteins with highly divergent functions in eicosanoid and glutathione metabolism”. Protein Sci. 8 (3): 689–92. doi:10.1110/ps.8.3.689. PMC 2144274. PMID 10091672.
- “Entrez Gene: PTGES prostaglandin E synthase”.
- Jegerschold, C.; Pawelzik, S. -C.; Purhonen, P.; Bhakat, P.; Gheorghe, K. R.; Gyobu, N.; Mitsuoka, K.; Morgenstern, R.; Jakobsson, P. -J.; Hebert, H. (2008). “Structural basis for induced formation of the inflammatory mediator prostaglandin E2”. Proceedings of the National Academy of Sciences. 105 (32): 11110–11115. Bibcode:2008PNAS..10511110J. doi:10.1073/pnas.0802894105. PMC 2516235. PMID 18682561.
- Murakami M, Nakatani Y, Tanioka T, Kudo I (August 2002). “Prostaglandin E synthase”. Prostaglandins Other Lipid Mediat. 68–69: 383–99. doi:10.1016/S0090-6980(02)00043-6. PMID 12432931. S2CID 22246484.
- Park JY, Pillinger MH, Abramson SB (June 2006). “Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases”. Clin. Immunol. 119 (3): 229–40. doi:10.1016/j.clim.2006.01.016. PMID 16540375.
External links
- prostaglandin-E+synthase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- EC 5.3.99.3
| Metabolism: lipid metabolism – eicosanoid metabolism enzymes |
|---|
| Isomerases: intramolecular oxidoreductases (EC 5.3) |
|---|
| Drugs for erectile dysfunction (G04BE) and premature ejaculation |
|---|
Further reading
- Bastien L, Sawyer N, Grygorczyk R, et al. (1994). “Cloning, functional expression, and characterization of the human prostaglandin E2 receptor EP2 subtype”. J. Biol. Chem. 269 (16): 11873–7. doi:10.1016/S0021-9258(17)32654-6. PMID 8163486.
- Wu T, Wu H, Wang J, Wang J (2011). “Expression and cellular localization of cyclooxygenases and prostaglandin E synthases in the hemorrhagic brain”. J Neuroinflammation. 8: 22. doi:10.1186/1742-2094-8-22. PMC 3062590. PMID 21385433.
- Funk CD, Furci L, FitzGerald GA, et al. (1994). “Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype”. J. Biol. Chem. 268 (35): 26767–72. doi:10.1016/S0021-9258(19)74379-8. PMID 8253813.
- Giacomini E, Giordani L, di Modugno F, et al. (1998). “Increased PGE2 production mediates the in vitro inhibitory effect of the human immunodeficiency virus P24 immunosuppressive heptapeptide Ch7”. Scand. J. Immunol. 48 (3): 248–53. doi:10.1046/j.1365-3083.1998.00389.x. PMID 9743208. S2CID 20332912.
- Jakobsson PJ, Thorén S, Morgenstern R, Samuelsson B (1999). “Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target”. Proc. Natl. Acad. Sci. U.S.A. 96 (13): 7220–5. Bibcode:1999PNAS…96.7220J. doi:10.1073/pnas.96.13.7220. PMC 22058. PMID 10377395.
- Forsberg L, Leeb L, Thorén S, et al. (2000). “Human glutathione dependent prostaglandin E synthase: gene structure and regulation”. FEBS Lett. 471 (1): 78–82. doi:10.1016/S0014-5793(00)01367-3. PMID 10760517. S2CID 10901322.
- Han R, Smith TJ (2002). “Cytoplasmic prostaglandin E2 synthase is dominantly expressed in cultured KAT-50 thyrocytes, cells that express constitutive prostaglandin-endoperoxide H synthase-2. Basis for low protaglandin E2 production”. J. Biol. Chem. 277 (39): 36897–903. doi:10.1074/jbc.M206949200. PMID 12145315.
- Giannoulias D, Alfaidy N, Holloway AC, et al. (2002). “Expression of prostaglandin I(2) synthase, but not prostaglandin E synthase, changes in myometrium of women at term pregnancy”. J. Clin. Endocrinol. Metab. 87 (11): 5274–82. doi:10.1210/jc.2002-020521. PMID 12414902.
- Ouellet M, Falgueyret JP, Ear PH, et al. (2003). “Purification and characterization of recombinant microsomal prostaglandin E synthase-1”. Protein Expr. Purif. 26 (3): 489–95. doi:10.1016/S1046-5928(02)00566-1. PMID 12460774.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). “Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences”. Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS…9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Uracz W, Uracz D, Olszanecki R, Gryglewski RJ (2003). “Interleukin 1beta induces functional prostaglandin E synthase in cultured human umbilical vein endothelial cells”. J. Physiol. Pharmacol. 53 (4 Pt 1): 643–54. PMID 12512699.
- Meadows JW, Eis AL, Brockman DE, Myatt L (2003). “Expression and localization of prostaglandin E synthase isoforms in human fetal membranes in term and preterm labor”. J. Clin. Endocrinol. Metab. 88 (1): 433–9. doi:10.1210/jc.2002-021061. PMID 12519887.
- Kamei D, Murakami M, Nakatani Y, et al. (2003). “Potential role of microsomal prostaglandin E synthase-1 in tumorigenesis”. J. Biol. Chem. 278 (21): 19396–405. doi:10.1074/jbc.M213290200. PMID 12626523.
- Jakobsson PJ, Thorén S, Morgenstern R, Samuelsson B (2003). “Characterization of Microsomal, Glutathione Dependent Prostaglandin e Synthase”. Eicosanoids and Other Bioactive Lipids in Cancer, Inflammation, and Radiation Injury, 5. Advances in Experimental Medicine and Biology. Vol. 507. pp. 287–91. doi:10.1007/978-1-4615-0193-0_44. ISBN 978-0-306-47283-1. PMID 12664599.
- Thorén S, Weinander R, Saha S, et al. (2003). “Human microsomal prostaglandin E synthase-1: purification, functional characterization, and projection structure determination”. J. Biol. Chem. 278 (25): 22199–209. doi:10.1074/jbc.M303227200. PMID 12672824.
- Alfaidy N, Sun M, Challis JR, Gibb W (2003). “Expression of membrane prostaglandin E synthase in human placenta and fetal membranes and effect of labor”. Endocrine. 20 (3): 219–25. doi:10.1385/ENDO:20:3:219. PMID 12721500. S2CID 9590845.
- Ekström L, Lyrenäs L, Jakobsson PJ, et al. (2003). “Basal expression of the human MAPEG members microsomal glutathione transferase 1 and prostaglandin E synthase genes is mediated by Sp1 and Sp3”. Biochim. Biophys. Acta. 1627 (2–3): 79–84. doi:10.1016/S0167-4781(03)00077-0. PMID 12818425.
- Murakami M, Nakashima K, Kamei D, et al. (2003). “Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2”. J. Biol. Chem. 278 (39): 37937–47. doi:10.1074/jbc.M305108200. PMID 12835322.
- Trebino CE, Stock JL, Gibbons CP, et al. (2003). “Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase”. Proc. Natl. Acad. Sci. U.S.A. 100 (15): 9044–9. Bibcode:2003PNAS..100.9044T. doi:10.1073/pnas.1332766100. PMC 166435. PMID 12835414.
- Johnson JL, Beito TG, Krco CJ, Toft DO (1994). “Characterization of a novel 23-kilodalton protein of inactive progesterone receptor complexes”. Mol. Cell. Biol. 14 (3): 1956–63. doi:10.1128/MCB.14.3.1956. PMC 358554. PMID 8114727.
- Wu T, Wu H, Wang J, Wang J (2011). “Expression and cellular localization of cyclooxygenases and prostaglandin E synthases in the hemorrhagic brain”. J Neuroinflammation. 8: 22. doi:10.1186/1742-2094-8-22. PMC 3062590. PMID 21385433.
- Bonaldo MF, Lennon G, Soares MB (1997). “Normalization and subtraction: two approaches to facilitate gene discovery”. Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Dittmar KD, Pratt WB (1997). “Folding of the glucocorticoid receptor by the reconstituted Hsp90-based chaperone machinery. The initial hsp90.p60.hsp70-dependent step is sufficient for creating the steroid binding conformation”. J. Biol. Chem. 272 (20): 13047–13054. doi:10.1074/jbc.272.20.13047. PMID 9148915.
- Dittmar KD, Demady DR, Stancato LF, et al. (1997). “Folding of the glucocorticoid receptor by the heat shock protein (hsp) 90-based chaperone machinery. The role of p23 is to stabilize receptor.hsp90 heterocomplexes formed by hsp90.p60.hsp70”. J. Biol. Chem. 272 (34): 21213–21220. doi:10.1074/jbc.272.34.21213. PMID 9261129.
- Zou J, Guo Y, Guettouche T, et al. (1998). “Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1”. Cell. 94 (4): 471–480. doi:10.1016/S0092-8674(00)81588-3. PMID 9727490. S2CID 9234420.
- Yoo JY, Hamburger AW (1999). “Interaction of the p23/p198 protein with ErbB-3”. Gene. 229 (1–2): 215–221. doi:10.1016/S0378-1119(98)00604-0. PMID 10095121.
- Knoblauch R, Garabedian MJ (1999). “Role for Hsp90-associated cochaperone p23 in estrogen receptor signal transduction”. Mol. Cell. Biol. 19 (5): 3748–59. doi:10.1128/MCB.19.5.3748. PMC 84199. PMID 10207098.
- Muñoz MJ, Bejarano ER, Daga RR, Jimenez J (2000). “The identification of Wos2, a p23 homologue that interacts with Wee1 and Cdc2 in the mitotic control of fission yeasts”. Genetics. 153 (4): 1561–72. doi:10.1093/genetics/153.4.1561. PMC 1460861. PMID 10581266.
- Freeman BC, Felts SJ, Toft DO, Yamamoto KR (2000). “The p23 molecular chaperones act at a late step in intracellular receptor action to differentially affect ligand efficacies”. Genes Dev. 14 (4): 422–34. doi:10.1101/gad.14.4.422. PMC 316379. PMID 10691735.
- Weaver AJ, Sullivan WP, Felts SJ, et al. (2000). “Crystal structure and activity of human p23, a heat shock protein 90 co-chaperone”. J. Biol. Chem. 275 (30): 23045–23052. doi:10.1074/jbc.M003410200. PMID 10811660.
- Tanioka T, Nakatani Y, Semmyo N, et al. (2000). “Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis”. J. Biol. Chem. 275 (42): 32775–32782. doi:10.1074/jbc.M003504200. PMID 10922363.
- Kazlauskas A, Poellinger L, Pongratz I (2001). “The immunophilin-like protein XAP2 regulates ubiquitination and subcellular localization of the dioxin receptor”. J. Biol. Chem. 275 (52): 41317–41324. doi:10.1074/jbc.M007765200. PMID 11013261.
- Futatsumori M, Kasai K, Takatsu H, et al. (2001). “Identification and characterization of novel isoforms of COP I subunits”. J. Biochem. 128 (5): 793–801. doi:10.1093/oxfordjournals.jbchem.a022817. PMID 11056392.
- Kazlauskas A, Sundström S, Poellinger L, Pongratz I (2001). “The hsp90 chaperone complex regulates intracellular localization of the dioxin receptor”. Mol. Cell. Biol. 21 (7): 2594–2607. doi:10.1128/MCB.21.7.2594-2607.2001. PMC 86890. PMID 11259606.
- Forsythe HL, Jarvis JL, Turner JW, et al. (2001). “Stable association of hsp90 and p23, but Not hsp70, with active human telomerase”. J. Biol. Chem. 276 (19): 15571–15574. doi:10.1074/jbc.C100055200. PMID 11274138.
- Donzé O, Abbas-Terki T, Picard D (2001). “The Hsp90 chaperone complex is both a facilitator and a repressor of the dsRNA-dependent kinase PKR”. EMBO J. 20 (14): 3771–3780. doi:10.1093/emboj/20.14.3771. PMC 125551. PMID 11447118.
- Elder RT, Yu M, Chen M, et al. (2001). “HIV-1 Vpr induces cell cycle G2 arrest in fission yeast (Schizosaccharomyces pombe) through a pathway involving regulatory and catalytic subunits of PP2A and acting on both Wee1 and Cdc25”. Virology. 287 (2): 359–370. doi:10.1006/viro.2001.1007. PMID 11531413.
- Hernández MP, Chadli A, Toft DO (2002). “HSP40 binding is the first step in the HSP90 chaperoning pathway for the progesterone receptor”. J. Biol. Chem. 277 (14): 11873–11881. doi:10.1074/jbc.M111445200. PMID 11809754.
- McLaughlin SH, Smith HW, Jackson SE (2002). “Stimulation of the weak ATPase activity of human hsp90 by a client protein”. J. Mol. Biol. 315 (4): 787–798. doi:10.1006/jmbi.2001.5245. PMID 11812147.
- Cox MB, Miller CA (2002). “The p23 co-chaperone facilitates dioxin receptor signaling in a yeast model system”. Toxicol. Lett. 129 (1–2): 13–21. doi:10.1016/S0378-4274(01)00465-9. PMID 11879970.
- Lehner B, Sanderson CM (Jul 2004). “A protein interaction framework for human mRNA degradation”. Genome Research. 14 (7): 1315–23. doi:10.1101/gr.2122004. PMC 442147. PMID 15231747.
- Palmieri RT, Wilson MA, Iversen ES, Clyde MA, Calingaert B, Moorman PG, Poole C, Anderson AR, Anderson S, Anton-Culver H, Beesley J, Hogdall E, Brewster W, Carney ME, Chen X, Chenevix-Trench G, Chang-Claude J, Cunningham JM, Dicioccio RA, Doherty JA, Easton DF, Edlund CK, Gayther SA, Gentry-Maharaj A, Goode EL, Goodman MT, Kjaer SK, Hogdall CK, Hopkins MP, Jenison EL, Blaakaer J, Lurie G, McGuire V, Menon U, Moysich KB, Ness RB, Pearce CL, Pharoah PD, Pike MC, Ramus SJ, Rossing MA, Song H, Terada KY, Vandenberg D, Vierkant RA, Wang-Gohrke S, Webb PM, Whittemore AS, Wu AH, Ziogas A, Berchuck A, Schildkraut JM (Dec 2008). “Polymorphism in the IL18 gene and epithelial ovarian cancer in non-Hispanic white women”. Cancer Epidemiology, Biomarkers & Prevention. 17 (12): 3567–72. doi:10.1158/1055-9965.EPI-08-0548. PMC 2664299. PMID 19064572.
- Chaudhry UA, Dore S (2009). “Cytosolic prostaglandin E synthase: expression patterns in control and Alzheimer’s disease brains”. American Journal of Alzheimer’s Disease & Other Dementias. 24 (1): 46–51. doi:10.1177/1533317508323655. PMC 2859688. PMID 19001348.
- Wu T, Wu H, Wang J, Wang J (2011). “Expression and cellular localization of cyclooxygenases and prostaglandin E synthases in the hemorrhagic brain”. Journal of Neuroinflammation. 8: 22. doi:10.1186/1742-2094-8-22. PMC 3062590. PMID 21385433.
- Dagle JM, Lepp NT, Cooper ME, Schaa KL, Kelsey KJ, Orr KL, Caprau D, Zimmerman CR, Steffen KM, Johnson KJ, Marazita ML, Murray JC (Apr 2009). “Determination of genetic predisposition to patent ductus arteriosus in preterm infants”. Pediatrics. 123 (4): 1116–23. doi:10.1542/peds.2008-0313. PMC 2734952. PMID 19336370.
- Fischer A, Grallert H, Böhme M, Gieger C, Boomgaarden I, Heid I, Wichmann HE, Döring F, Illig T (Apr 2009). “Association analysis between the prostaglandin E synthase 2 R298H polymorphism and body mass index in 8079 participants of the KORA study cohort”. Genetic Testing and Molecular Biomarkers. 13 (2): 223–6. doi:10.1089/gtmb.2008.0111. PMID 19371221.
- Mattila S, Tuominen H, Koivukangas J, Stenbäck F (Apr 2009). “The terminal prostaglandin synthases mPGES-1, mPGES-2, and cPGES are all overexpressed in human gliomas”. Neuropathology. 29 (2): 156–65. doi:10.1111/j.1440-1789.2008.00963.x. PMID 19347995. S2CID 22681905.
- Lindner I, Helwig U, Rubin D, Fischer A, Marten B, Schreiber S, Döring F, Schrezenmeir J (Dec 2007). “Prostaglandin E synthase 2 (PTGES2) Arg298His polymorphism and parameters of the metabolic syndrome”. Molecular Nutrition & Food Research. 51 (12): 1447–51. doi:10.1002/mnfr.200700144. PMID 17979097.
- Seo T, Tatsuguchi A, Shinji S, Yonezawa M, Mitsui K, Tanaka S, Fujimori S, Gudis K, Fukuda Y, Sakamoto C (Jun 2009). “Microsomal prostaglandin E synthase protein levels correlate with prognosis in colorectal cancer patients”. Virchows Archiv. 454 (6): 667–76. doi:10.1007/s00428-009-0777-z. PMID 19412621. S2CID 6233883.
- Lee E, Choi MK, Lee YJ, Ku JL, Kim KH, Choi JS, Lim SJ (Nov 2006). “Alpha-tocopheryl succinate, in contrast to alpha-tocopherol and alpha-tocopheryl acetate, inhibits prostaglandin E2 production in human lung epithelial cells”. Carcinogenesis. 27 (11): 2308–15. doi:10.1093/carcin/bgl073. PMID 16714329.
- Meng Q, Raha A, Roy S, Hu J, Kalvakolanu DV (May 2005). “IFN-gamma-stimulated transcriptional activation by IFN-gamma-activated transcriptional element-binding factor 1 occurs via an inducible interaction with CAAAT/enhancer-binding protein-beta”. Journal of Immunology. 174 (10): 6203–11. doi:10.4049/jimmunol.174.10.6203. PMID 15879117.
- Mehrle A, Rosenfelder H, Schupp I, del Val C, Arlt D, Hahne F, Bechtel S, Simpson J, Hofmann O, Hide W, Glatting KH, Huber W, Pepperkok R, Poustka A, Wiemann S (Jan 2006). “The LIFEdb database in 2006”. Nucleic Acids Research. 34 (Database issue): D415–8. doi:10.1093/nar/gkj139. PMC 1347501. PMID 16381901.
- Oh JH, Yang JO, Hahn Y, Kim MR, Byun SS, Jeon YJ, Kim JM, Song KS, Noh SM, Kim S, Yoo HS, Kim YS, Kim NS (Dec 2005). “Transcriptome analysis of human gastric cancer”. Mammalian Genome. 16 (12): 942–54. doi:10.1007/s00335-005-0075-2. PMID 16341674. S2CID 69278.
- Wiemann S, Arlt D, Huber W, Wellenreuther R, Schleeger S, Mehrle A, Bechtel S, Sauermann M, Korf U, Pepperkok R, Sültmann H, Poustka A (Oct 2004). “From ORFeome to biology: a functional genomics pipeline”. Genome Research. 14 (10B): 2136–44. doi:10.1101/gr.2576704. PMC 528930. PMID 15489336.
- Chaudhry UA, Zhuang H, Crain BJ, Doré S (Jan 2008). “Elevated microsomal prostaglandin-E synthase-1 in Alzheimer’s disease”. Alzheimer’s & Dementia. 4 (1): 6–13. doi:10.1016/j.jalz.2007.10.015. PMC 2500207. PMID 18631945.
- Nitz I, Fisher E, Grallert H, Li Y, Gieger C, Rubin D, Boeing H, Spranger J, Lindner I, Schreiber S, Rathmann W, Gohlke H, Döring A, Wichmann HE, Schrezenmeir J, Döring F, Illig T (Aug 2007). “Association of prostaglandin E synthase 2 (PTGES2) Arg298His polymorphism with type 2 diabetes in two German study populations”. The Journal of Clinical Endocrinology and Metabolism. 92 (8): 3183–8. doi:10.1210/jc.2006-2550. PMID 17566096.
- Colombe L, Vindrios A, Michelet JF, Bernard BA (Sep 2007). “Prostaglandin metabolism in human hair follicle”. Experimental Dermatology. 16 (9): 762–9. doi:10.1111/j.1600-0625.2007.00586.x. PMID 17697149. S2CID 43663330.
- Fisher E, Nitz I, Lindner I, Rubin D, Boeing H, Möhlig M, Hampe J, Schreiber S, Schrezenmeir J, Döring F (Feb 2007). “Candidate gene association study of type 2 diabetes in a nested case-control study of the EPIC-Potsdam cohort – role of fat assimilation”. Molecular Nutrition & Food Research. 51 (2): 185–91. doi:10.1002/mnfr.200600162. PMID 17266179.

Leave a Reply