Staphylokinase (SAK; also known as staphylococcal fibrinolysin or Müller’s factor) is a protein produced by Staphylococcus aureus. It contains 136 amino acid residues and has a molecular mass of 15kDa. Synthesis of staphylokinase occurs in late exponential phase. It is similar to streptokinase.
- Bokarewa MI, Jin T, Tarkowski A (2006). “Staphylococcus aureus: Staphylokinase”. The International Journal of Biochemistry & Cell Biology. 38 (4): 504–509. doi:10.1016/j.biocel.2005.07.005. PMID 16111912.
Fibrinolysin is an enzyme derived from plasma of bovine origin (plasmin) or extracted from cultures of certain bacteria. (more below)
Staphylokinase is positively regulated by the “agr” gene regulator. It activates plasminogen to form plasmin, which digests fibrin clots. This disrupts the fibrin meshwork which forms to keep infections localized. Staphylokinase interacts with plasminogen to form a 1:1 complex that exposes the active site of the plasminogen molecule. The plasmin Sak complex is neutralized by α2– antiplasmin in plasma in the absence of fibrin, resulting in lysis. However, in the presence of fibrin, the inhibition is delayed, creating a unique mechanism for fibrin selectivity in plasma.[citation needed]
- Bokarewa MI, Jin T, Tarkowski A (2006). “Staphylococcus aureus: Staphylokinase”. The International Journal of Biochemistry & Cell Biology. 38 (4): 504–509. doi:10.1016/j.biocel.2005.07.005. PMID 16111912.
Plasmin is an important enzyme (EC3.4.21.7) present in blood that degrades many blood plasma proteins, including fibrinclots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein (in the zymogen form of plasminogen) is encoded by the PLGgene. Plasmin is a serine protease that acts to dissolve fibrin blood clots. Apart from fibrinolysis, plasmin proteolyses proteins in various other systems: It activates collagenases, some mediators of the complement system, and weakens the wall of the Graafian follicle, leading to ovulation. Plasmin is also integrally involved in inflammation. It cleaves fibrin, fibronectin, thrombospondin, laminin, and von Willebrand factor. Plasmin, like trypsin, belongs to the family of serine proteases. Plasmin is released as a zymogen called plasminogen (PLG) from the liver into the systemic circulation. Two major glycoforms of plasminogen are present in humans – type I plasminogen contains two glycosylation moieties (N-linked to N289 and O-linked to T346), whereas type II plasminogen contains only a single O-linked sugar (O-linked to T346). Type II plasminogen is preferentially recruited to the cell surface over the type I glycoform. Conversely, type I plasminogen appears more readily recruited to blood clots. In circulation, plasminogen adopts a closed, activation-resistant conformation. Upon binding to clots, or to the cell surface, plasminogen adopts an open form that can be converted into active plasmin by a variety of enzymes, including tissue plasminogen activator (tPA), urokinase plasminogen activator (uPA), kallikrein, and factor XII (Hageman factor). Fibrin is a cofactor for plasminogen activation by tissue plasminogen activator. Urokinase plasminogen activator receptor (uPAR) is a cofactor for plasminogen activation by urokinase plasminogen activator. The conversion of plasminogen to plasmin involves the cleavage of the peptide bond between Arg-561 and Val-562. Plasmin cleavage produces angiostatin.
- “Entrez Gene: plasminogen”.
- Atsev S, Tomov N (December 2020). “Using antifibrinolytics to tackle neuroinflammation”. Neural Regeneration Research. 15 (12): 2203–2206. doi:10.4103/1673-5374.284979. PMC 7749481. PMID 32594031.
- Miyata T, Iwanaga S, Sakata Y, Aoki N (October 1982). “Plasminogen Tochigi: inactive plasmin resulting from replacement of alanine-600 by threonine in the active site”. Proceedings of the National Academy of Sciences of the United States of America. 79 (20): 6132–6136. Bibcode:1982PNAS…79.6132M. doi:10.1073/pnas.79.20.6132. PMC 347073. PMID 6216475.
- Forsgren M, Råden B, Israelsson M, Larsson K, Hedén LO (March 1987). “Molecular cloning and characterization of a full-length cDNA clone for human plasminogen”. FEBS Letters. 213 (2): 254–260. doi:10.1016/0014-5793(87)81501-6. PMID 3030813. S2CID 9075872.
- Law RH, Caradoc-Davies T, Cowieson N, Horvath AJ, Quek AJ, Encarnacao JA, et al. (March 2012). “The X-ray crystal structure of full-length human plasminogen”. Cell Reports. 1 (3): 185–190. doi:10.1016/j.celrep.2012.02.012. PMID 22832192.
Staphylokinase also cleaves IgG and complement component C3b, inhibiting phagocytosis.
- Rooijakkers, SH; van Wamel, WJ; Ruyken, M; van Kessel, KP; van Strijp, JA (March 2005). “Anti-opsonic properties of staphylokinase”. Microbes and Infection. 7 (3): 476–84. doi:10.1016/j.micinf.2004.12.014. PMID 15792635.
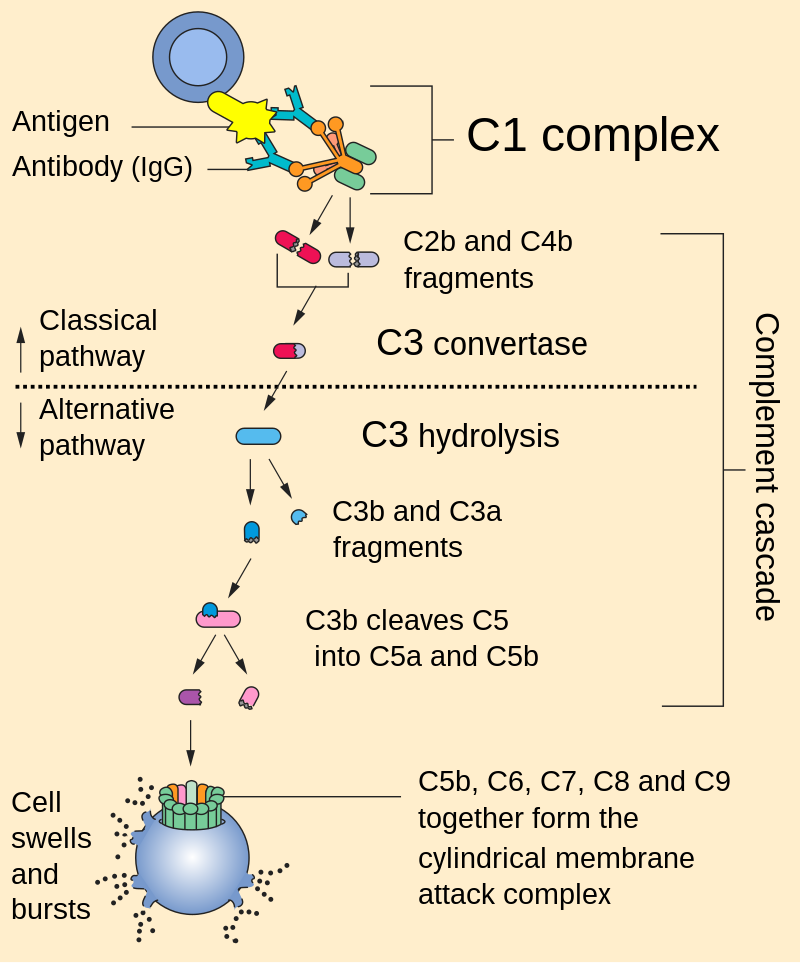
C3b is the larger of two elements formed by the cleavage of complement component 3, and is considered an important part of the innate immune system. C3b is potent in opsonization: tagging pathogens, immune complexes (antigen–antibody), and apoptotic cells for phagocytosis. Additionally, C3b plays a role in forming a C3 convertase when bound to Factor B (C3bBb complex), or a C5 convertase when bound to C4b and C2b (C4b2b3b complex) or when an additional C3b molecule binds to the C3bBb complex (C3bBb3b complex).
- Liszewski, M. Kathryn; Atkinson, John P. (2015-06-10). “Complement regulator CD46: genetic variants and disease associations”. Human Genomics. 9 (1): 7. doi:10.1186/s40246-015-0029-z. PMC 4469999. PMID 26054645.
C3b’s ability to perform these important functions derives from its ability to covalently bind to the surface of invading pathogens within an organism’s body. The cleavage of C3 leaves C3b with an exposed thioester bond, allowing C3b to effectively coat and tag foreign cells by covalently binding to hydroxyl (-OH) and amine (-NH2) groups on foreign cell surfaces.
- Merle, Nicolas S.; Noe, Remi; Halbwachs-Mecarelli, Lise; Fremeaux-Bacchi, Veronique; Roumenina, Lubka T. (2015-05-26). “Complement System Part II: Role in Immunity”. Frontiers in Immunology. 6: 257. doi:10.3389/fimmu.2015.00257. ISSN 1664-3224. PMC 4443744. PMID 26074922.
This cleavage can occur via three mechanisms (classical pathway, alternative pathway and lectin pathway) that ultimately lead to the formation of a C3 convertase. Formation of a C3 convertase functions as a positive feedback loop, so as more C3b is cleaved, more C3 convertases are formed, further amplifying the signal on the surface of the microbial invader. This amplification of signal serves as a powerful tool for the immune system in effective clearance of the invading pathogen.
Structure
The full length of mature staphylokinase mRNA is 489bp. The first 27 amino acids code for a signal peptide which is cleaved off in the mature protein (mSak). There is little or no homology between the primary structure of Sak and other plasminogen activators. The natural variants of Sak are Sak42D, SakφC and SakSTAR. These variants have four nucleotide differences in the coding region with one silent mutation. The affected codons are amino acids 38, 61, 63 and 70 in the full length staphylokinase. Amino acid 38 is lysine, amino acid 61 is serine in SaKSTAR, glycine in SakφC, and arginine in Sak42D. Amino acid 63 is glycine in SakSTAR and SakφC, but arginine in Sak42D. SakSTAR and SakφC amino acid 70 is histidine, whereas it is arginine in Sak42D.
- Collen D, Lijnen HR (1994). “Staphylokinase, a fibrin-specific plasminogen activator with therapeutic potential?” (PDF). Blood. 84 (3): 680–686. doi:10.1182/blood.V84.3.680.680. PMID 7519069.
- Vanderschueren S, Van de Werf F, Collen D (August 1997). “Recombinant staphylokinase for thrombolytic therapy”. Fibrinolysis and Proteolysis. 11: 39–44. doi:10.1016/S0268-9499(97)80069-0.
The mature structure of staphylokinase consists of 163 amino acids, and it is elongated in shape. Sak contains two folded domains which are of similar size. The distance from the center of gravity between the two domains is 3.7 nm. When in solution, this position varies between the two domains suggesting a flexible dumbbell shape.[citation needed]
See also
Fibrinolysin is an enzyme derived from plasma of bovine origin (plasmin) or extracted from cultures of certain bacteria. It is used locally only and exclusively together with the enzyme desoxyribonuclease (extracted from bovine pancreas). Fibrinolysin and desoxyribonuclease both act as lytic enzymes. The combination is available as ointment containing 1 BU (Biological Unit) fibrinolysin and 666 BUs desoxyribonuclease per gram.
Fibrinolysin attacks and inactivates fibrin molecules occurring in undesirable exudates on the surface of the human body and on human mucosa, e.g., in superficial wounds and burns, while desoxyribonuclease targets and destroys (human) DNA. The combination of the two enzymes has a synergistic effect on necrotic but not on living tissue. According to the manufacturer the ointment provides enhanced wound cleaning and accelerates the healing process.
Both enzymes are marginally resorbed into systemic circulation because of their very high molecular weight and their macromolecular structure.
The activity of both enzymes is almost completely exhausted after 24 hours. Usually, it is necessary to repeat the application (and renew the dressing) every 6 to 8 hours until healing becomes complete.
The ointment is marketed by Pfizer under the brand name Fibrolan in a variety of countries (e.g. Switzerland). It is currently not approved in the USA.
Where approved, Fibrolan has been licensed on the basis of claimed good therapeutical experience, but adequate and well controlled studies are still lacking.
In the past, combinations with the antibiotic chloramphenicol were available, but because chloramphenicol in any form of application has led to aplastic anemia and death, these were banned. Additionally, combinations with the antifibrinolytic agent tranexamic acid have been withdrawn from pharmaceutic markets.
Indications
Enzymatic wound cleaning to assist healing of minor burns, superficial wounds, ulcus cruris, and superficial hematoma.
Contraindications and Precautions
The ointment should not be used in patients with a known hypersensitivity to any ingredient. It should be used with caution in patients with hypersensitivity to bovine proteins in general and in pregnant women (category C), because no human data is available.
Side-Effects
Infrequently, local reactions such as increased pain or a stitching/burning sensation are noticed. No systemic anticoagulant activity has been seen due to the exclusively local character of treatment.
Interactions
Not known.
See also
External links
- Swiss scientific product information
- Birk Y, Khalef S, Jibson MD (September 1983). “Purification and properties of protease F, a bacterial enzyme with chymotrypsin and elastase specificities”. Arch. Biochem. Biophys. 225 (2): 451–7. doi:10.1016/0003-9861(83)90053-X. PMID 6226244.
References
- Bokarewa MI, Jin T, Tarkowski A (2006). “Staphylococcus aureus: Staphylokinase”. The International Journal of Biochemistry & Cell Biology. 38 (4): 504–509. doi:10.1016/j.biocel.2005.07.005. PMID 16111912.
- Rooijakkers, SH; van Wamel, WJ; Ruyken, M; van Kessel, KP; van Strijp, JA (March 2005). “Anti-opsonic properties of staphylokinase”. Microbes and Infection. 7 (3): 476–84. doi:10.1016/j.micinf.2004.12.014. PMID 15792635.
- Collen D, Lijnen HR (1994). “Staphylokinase, a fibrin-specific plasminogen activator with therapeutic potential?” (PDF). Blood. 84 (3): 680–686. doi:10.1182/blood.V84.3.680.680. PMID 7519069.
- Vanderschueren S, Van de Werf F, Collen D (August 1997). “Recombinant staphylokinase for thrombolytic therapy”. Fibrinolysis and Proteolysis. 11: 39–44. doi:10.1016/S0268-9499(97)80069-0.
Leave a Reply