Juvenile hormone (and Methoprene)
Juvenile hormones (JHs) are a group of acyclic sesquiterpenoids that regulate many aspects of insect physiology. The first discovery of a JH was by Vincent Wigglesworth. JHs regulate development, reproduction, diapause, and polyphenisms. The chemical formula for juvenile hormone is C18H30O3. Riddiford, L. M. (1994). "Cellular and molecular actions
The Yolk’s On Us: Unraveling the Secrets of Vitellogenesis
Diagram of vitellogenesis in the digenean Crepidostomum metoecus. GER: granular endoplasmic reticulum; L: lipid droplet; M: mitochondrion; N: nucleus; Nl: nucleolus; SG: shell globule; SGC: shell globule cluster. Greani S, Quilichini Y, Marchand B (2016). "Ultrastructural study of vitellogenesis and oogenesis of Crepidostomum metoecus
The PVN Powerhouse: How the Paraventricular Nucleus Rules Hormone Secretion
Hold onto your hypothalamus, folks! We're about to dive into the wild world of the Paraventricular Nucleus (PVN), where tiny cells pack a mighty hormonal punch! Picture this: deep in the brain's control center, the hypothalamus, sits the PVN - a
Vitellogenin is a precursor of egg yolk that transports protein and some lipid from the liver through the blood to the growing oocytes where it becomes part of the yolk. Normally, it is only found in the blood or hemolymph of females…
Vitellogenin (VTG or less popularly known as VG) (from Latin vitellus, yolk, and genero, I produce) is a precursor of egg yolk that transports protein and some lipid from the liver through the blood to the growing oocytes where it becomes part of the yolk. Normally,
Vitellin is essential in the fertilization process, and embryonic development in egg-laying organisms
Vitellin is a protein found in the egg yolk. It is a phosphoprotein. Vitellin is a generic name for major of many yolk proteins. Vitellins at the U.S. National Library of Medicine Medical Subject Headings (MeSH) KUNKEL, JOSEPH G.; JOHN H. NORDIN (1985). "Yolk Proteins. 2.2 Vitellin, the major
Protein phosphorylation was first reported in 1906
Protein phosphorylation is a reversible post-translational modification of proteins in which an amino acid residue is phosphorylated by a protein kinase by the addition of a covalently bound phosphate group. Phosphorylation alters the structural conformation of a protein, causing it to become activated, deactivated, or otherwise modifying its function. Approximately 13,000 human proteins have
Phosphorylation
In biochemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion.[1] This process and its inverse, dephosphorylation, are common in biology.[2] Protein phosphorylation often activates (or deactivates) many enzymes.[3][4] During respiration and photosynthesis Phosphorylation is essential to the processes of both anaerobic and aerobic respiration, which involve the production of adenosine triphosphate (ATP),
Ku is a dimeric protein complex that binds to DNA double-strand break ends and is required for the non-homologous end joining (NHEJ) pathway of DNA repair
Ku is evolutionarily conserved from bacteria to humans. The ancestral bacterial Ku is a homodimer (two copies of the same protein bound to each other).[2] Eukaryotic Ku is a heterodimer of two polypeptides, Ku70 (XRCC6) and Ku80 (XRCC5), so named because the molecular weight of the human Ku proteins is around 70
Ku70
Ku70 is a protein that, in humans, is encoded by the XRCC6 gene.[5][6] Function Together, Ku70 and Ku80 make up the Ku heterodimer, which binds to DNA double-strand break ends and is required for the non-homologous end joining (NHEJ) pathway of DNA repair. It is also required for V(D)J recombination, which utilizes the NHEJ pathway to promote antigen
Ku80
Ku80 is a protein that, in humans, is encoded by the XRCC5 gene.[5] Together, Ku70 and Ku80 make up the Ku heterodimer, which binds to DNA double-strand break ends and is required for the non-homologous end joining (NHEJ) pathway of DNA repair. It is also required for V(D)J recombination, which utilizes the NHEJ pathway to promote antigen
Big Page of Hazel
The oldest confirmed hazel species is Corylus johnsonii found as fossils in the Ypresian-age rocks of Ferry County, Washington, USA
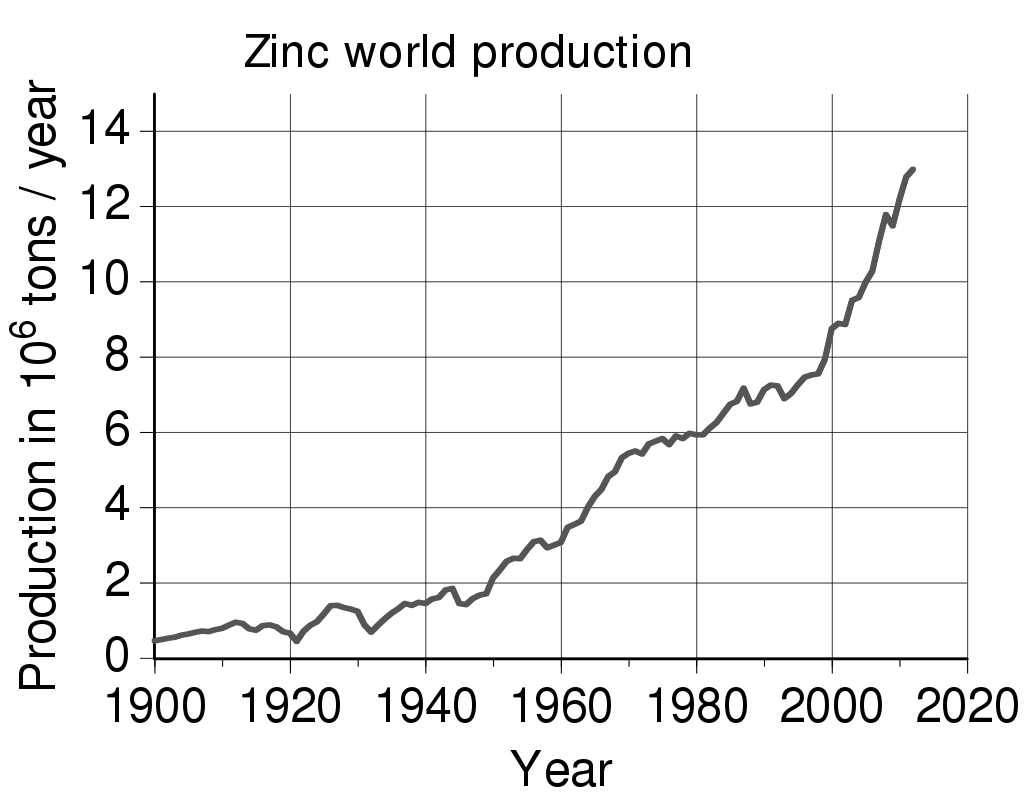
Zinc, maybe
No proof for the need of zinc in human cells was shown until the late 1930s. More than most ever wanted to know about zinc. A work in progress.
The trefoil knot fold is a protein fold in which the protein backbone is twisted into a trefoil knot shape
"Shallow" knots in which the tail of the polypeptide chain only passes through a loop by a few residues are uncommon, but "deep" knots in which many residues are passed through the loop are extremely rare. Deep trefoil knots have
The birds and the bees and the blister beetles
What on earth?
Railroad worms, glowworm beetles and apple maggots
Railroad worm with both lights on and off Railroad wormPhengodidae PhrixothrixScientific classificationDomain:EukaryotaKingdom:AnimaliaPhylum:ArthropodaClass:InsectaOrder:ColeopteraFamily:PhengodidaeGenus:PhrixothrixOlivier, 1909Species(several) A railroad worm is a larva or larviform female adult of a beetle of the genus Phrixothrix in the family Phengodidae, characterized by the possession of two different colors of bioluminescence. It has the appearance of a caterpillar. The eleven pairs of luminescent organs on
Cyanamide notes (it was a polio vaccine that spurred these notes and by now polio has five mentions on the page and these are two of them)
I'm going to add some polio vaccine stuff at the top of these notes. Hilary Koprowski is the one mentioned on the Polio Hall of Fame page who was not included in the hideous monument, see What In God's Name,