Category: Serine/Threonine
-
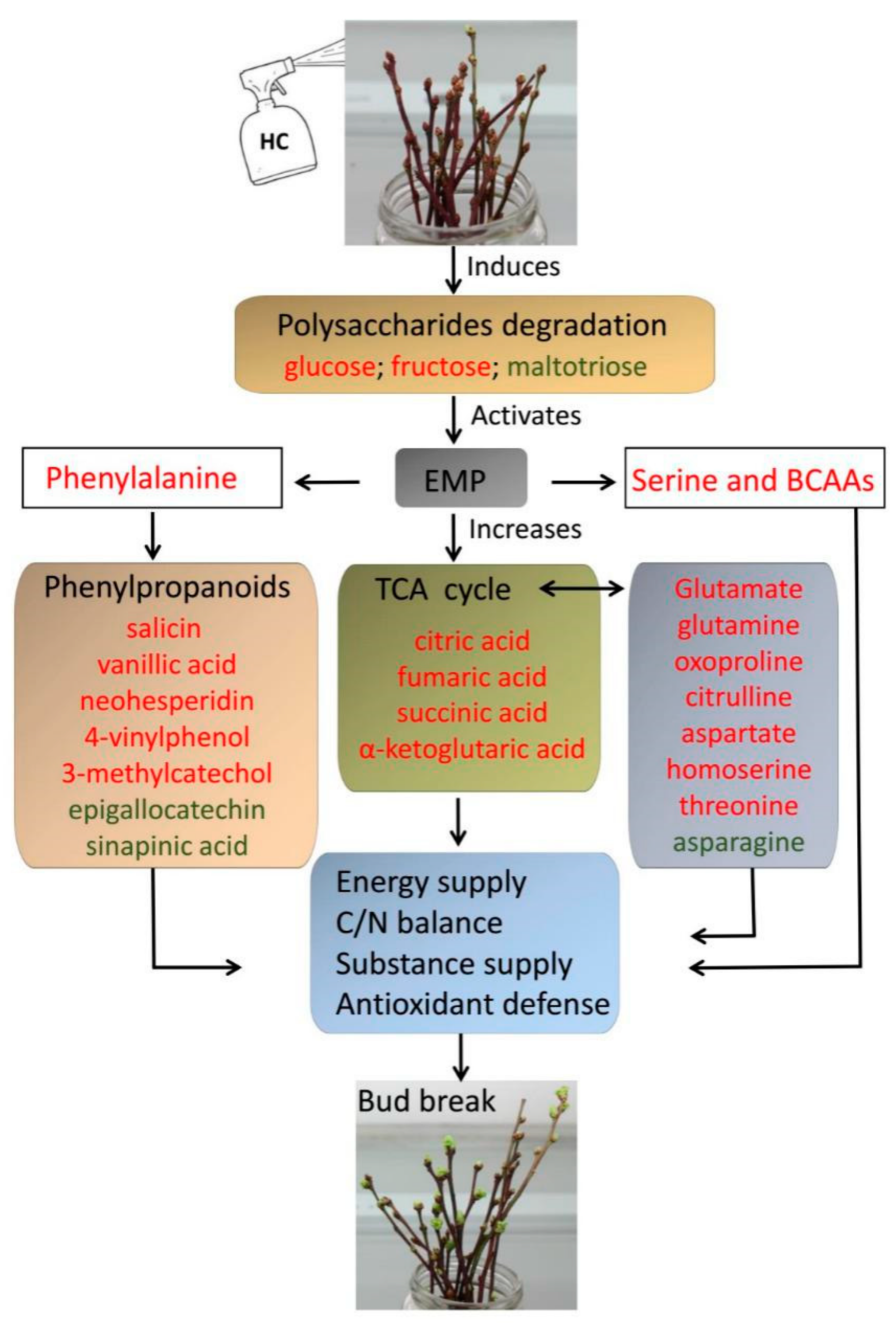
Cyanamide notes (it was a polio vaccine that spurred these notes and by now polio has five mentions on the page and these are two of them)
I’m going to add some polio vaccine stuff at the top of these notes. Hilary Koprowski is the one mentioned on the Polio Hall of Fame page who was not included in the hideous monument, see What In God’s Name, even though he (and his work) have direct connection to those who are included. He…
-
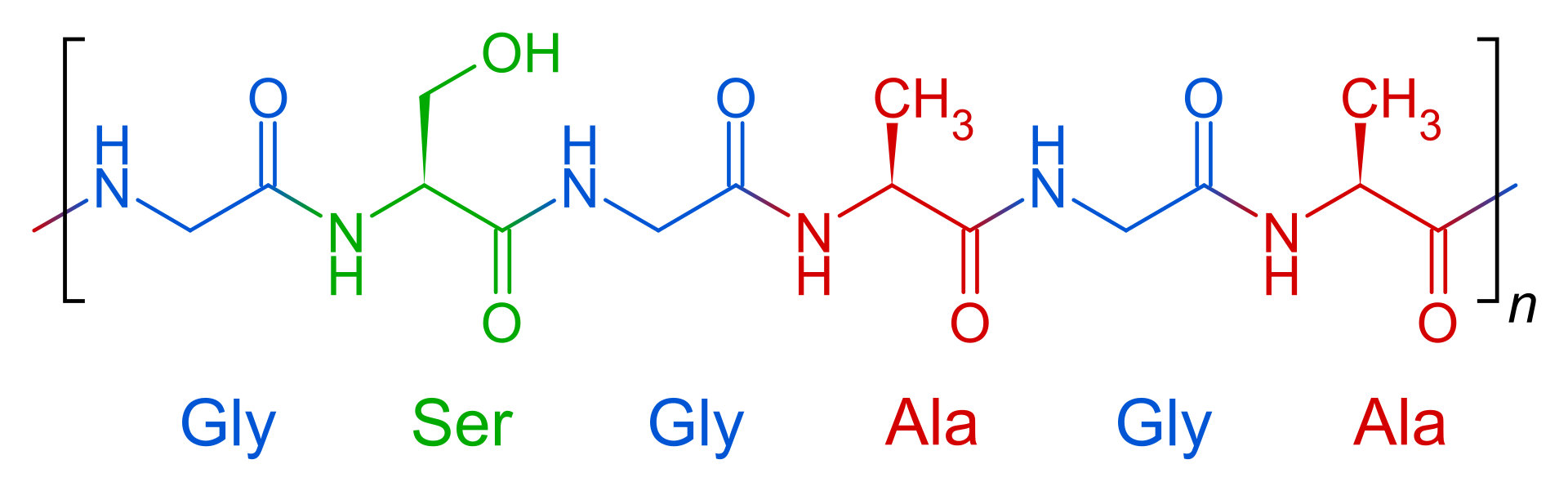
Fibroin is an insoluble protein present in silk produced by numerous insects
Fibroin is an insoluble protein present in silk produced by numerous insects, such as the larvae of Bombyx mori, and other moth genera such as Antheraea, Cricula, Samia and Gonometa. Silk in its raw state consists of two main proteins, sericin and fibroin, with a glue-like layer of sericin coating two singular filaments of fibroin called brins. BRIN AND BAVE (BRIN) One of the radiating sticks of a fan.…
-

Sericin is a protein created by Bombyx mori (silkworms) in the production of silk
Silk is a fibre produced by the silkworm in production of its cocoon. It consists mainly of two proteins, fibroin and sericin. Silk consists of 70–80% fibroin and 20–30% sericin; fibroin being the structural center of the silk, and sericin being the gum coating the fibres and allowing them to stick to each other. Structure Sericin is composed…
-
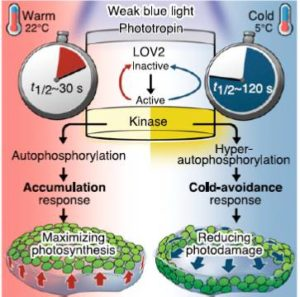
Phototropins are part of the phototropic sensory system in plants that causes various environmental responses in plants
Phototropins are photoreceptor proteins (more specifically, flavoproteins) that mediate phototropism responses in various species of algae, fungi and higher plants. Note: Flavoproteins are proteins that contain a nucleic acid derivative of riboflavin. These proteins are involved in a wide array of biological processes, including removal of radicals contributing to oxidative stress, photosynthesis, and DNA repair. The flavoproteins are some of the most-studied families of enzymes. Flavoproteins have either FMN (flavin mononucleotide) or FAD…
-
Fischer-Fantuzzi L, Vesco C. Deletion of 43 amino acids in the NH2-terminal half of the large tumor antigen of simian virus 40 results in a non-karyophilic protein capable of transforming established cells. Proc Natl Acad Sci U S A. 1985
Abstract We have characterized a simian virus 40 (SV40) mutant, derived from the viral DNA insertion present in simian cell transformants, which carries a deletion affecting the NH2-terminal region of the SV40 large tumor antigen. This mutant protein is 6% smaller than normal, has lost the typical nuclear localization of the SV40 large tumor antigen,…
-
Proline and a few other notes
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group -NH2 but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The “side chain” from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it…
-
Serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+3 form under biological conditions), a carboxyl group (which is in the deprotonated −COO− form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making…
-
Xylose is the first saccharide added to the serine or threonine in the proteoglycan type O-glycosylation
Xylose is the first saccharide added to the serine or threonine in the proteoglycan type O-glycosylation, and, so, it is the first saccharide in biosynthetic pathways of most anionic polysaccharides such as heparan sulfate and chondroitin sulfate. Definitions Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a “core protein” with one or more covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through…
-
Carboxypeptidases function in blood clotting, growth factor production, wound healing, reproduction, and many other processes
A carboxypeptidase (EC number 3.4.16 – 3.4.18) is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds at the N-terminus of proteins. Humans, animals, bacteria and plants contain several types of carboxypeptidases that have diverse functions ranging from catabolism to protein maturation. At least two mechanisms have been discussed. Functions Initial studies…
Recent Posts
- 🧬 Disease Table with Low Sodium Connection
- 🧂 Sodium Reduction and Sodium Replacement: A History of Reformulation and Exploding Diseases, Including Many Diseases Unheard of Before Deadly Sodium Policies
- 🧂 The DEADLY 1500 mg Sodium Recommendation predates the WHO’s formal global sodium reduction push by nearly a decade (and it’s even worse than that)
- 🧬 What Is Beta-Glucuronidase?
- When Sugar Was Salt: Crystalline Confusion and the Covenant of Sweetness
Tags
ADAM ASPARTAME Birds Blood Bones Brain Bugs Cancer Columba Cows crystallography Death Death cults Eggs Etymology Gastrin Gold Growth hormone History Hormones Insulin Liver Mere Perplexity Metal Monkey Business Mythology Paracetamol Plants Poison Pregnancy Protein Religion Reproduction Rocks Salt Slavery Snakes Sodium the birds and the bees Thiocyanate Tobacco Tylenol Underworld Venom zinc
