In supramolecular assembly
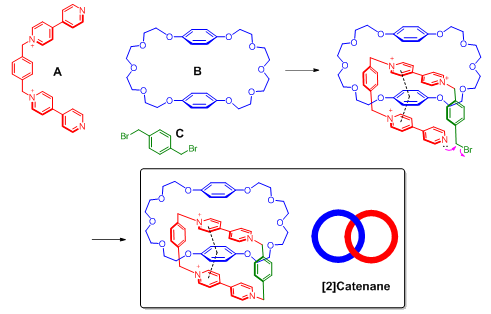
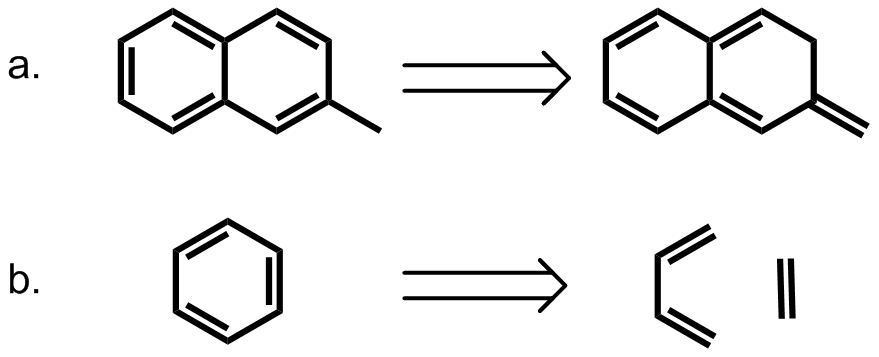
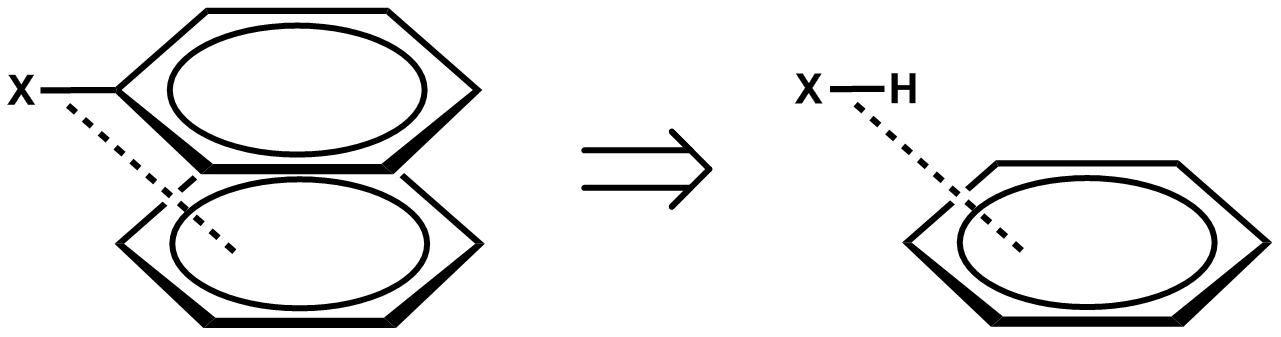
π systems are important building blocks in supramolecular assembly because of their versatile noncovalent interactions with various functional groups. A notable example of applying π–π interactions in supramolecular assembly is the synthesis of catenane. The major challenge for the synthesis
Addition in pharmacological active compounds
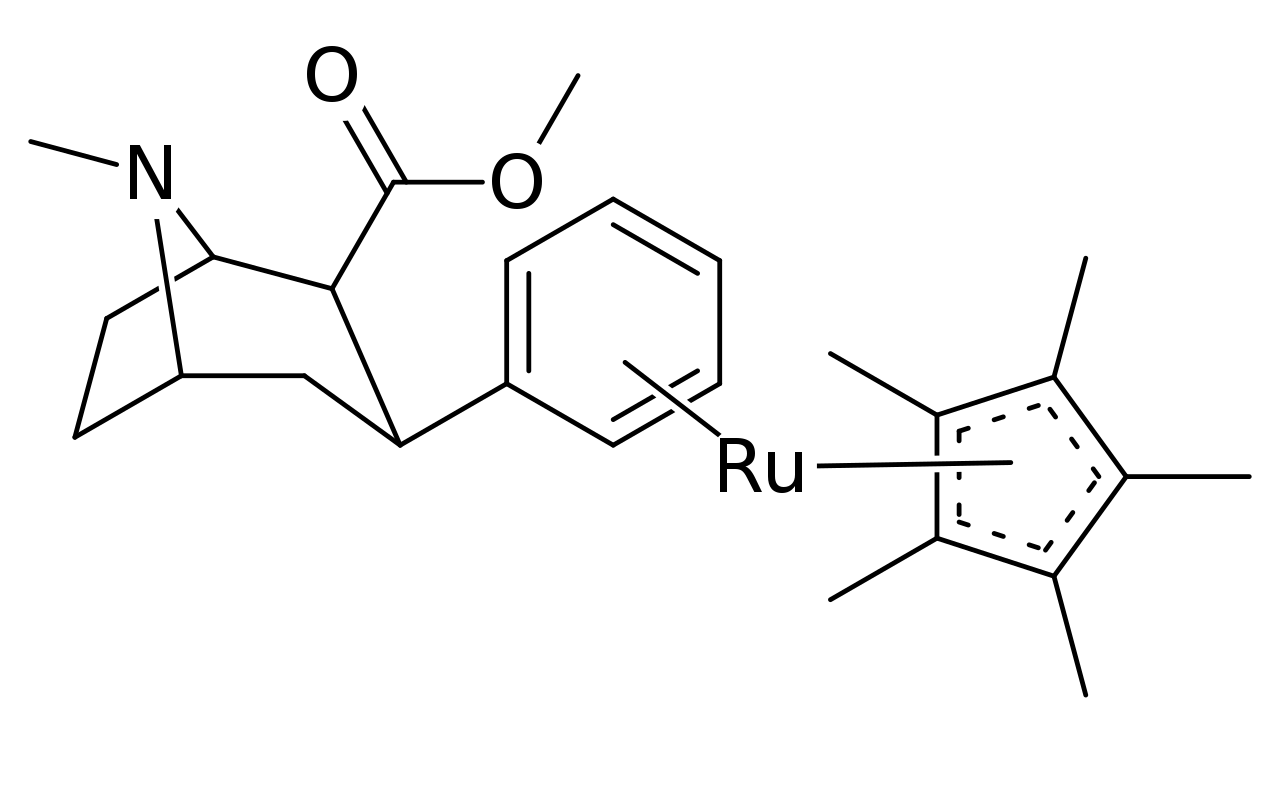
Several variants of pi coordinated phenyls have even been tested using transition metals for stacking η6-phenyltropanes, using cyclopentadienyl and tricarbonyl in place of a benzene. Which in the case of the tricarbonyl doubled the compound’s affinity for its intended ligand site (posited as
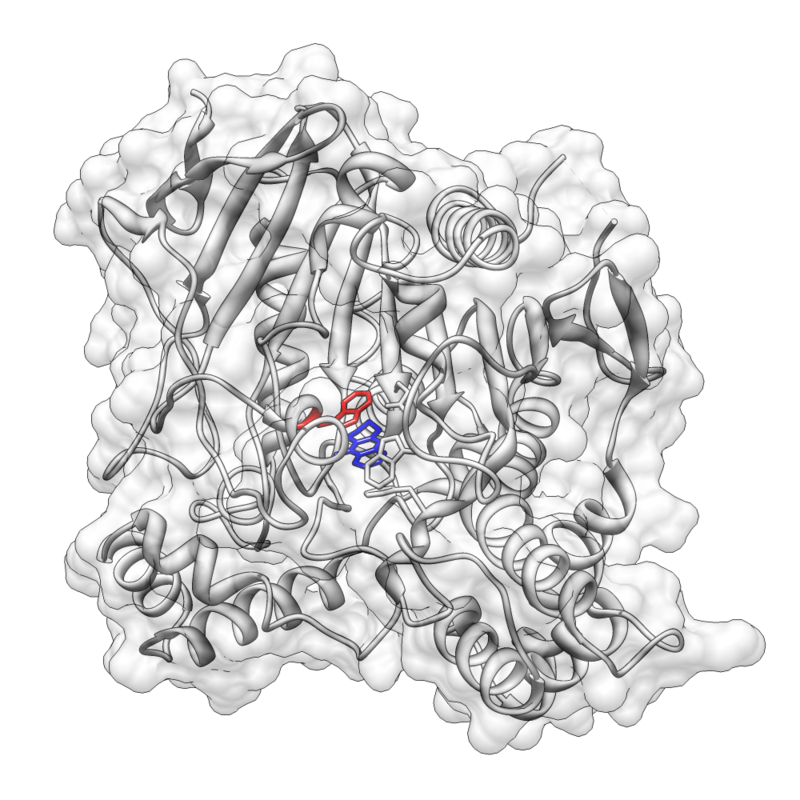
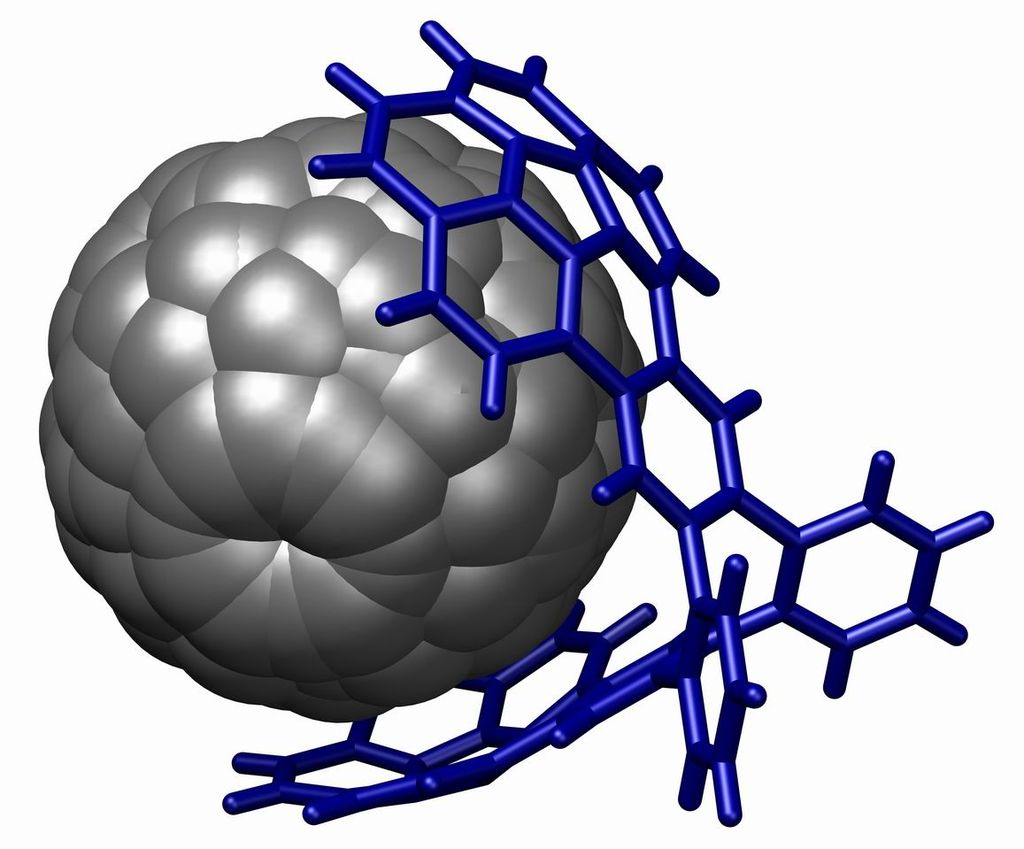
Pi stacking is prevalent in protein crystal structures and also contributes to the interactions between small molecules and proteins
As a result, pi–pi and cation–pi interactions are important factors in rational drug design. One example is the FDA-approved acetylcholinesterase (AChE) inhibitor tacrine which is used in the treatment of Alzheimer’s disease. Tacrine is proposed to have a pi stacking interaction with
Requirement of aromaticity
The conventional understanding of pi stacking involves quadrupole interactions between delocalized electrons in p-orbitals. In other words, aromaticity should be required for this interaction to occur. However, several groups have provided contrary evidence, calling into question whether pi stacking
Direct interaction model (substituent effects)
The Hunter–Sanders model has been criticized by numerous research groups offering contradictory experimental and computational evidence of pi stacking interactions that are not governed primarily by electrostatic effects. The clearest experimental evidence against electrostatic substituent effects
Electrostatic model (substituent effects)
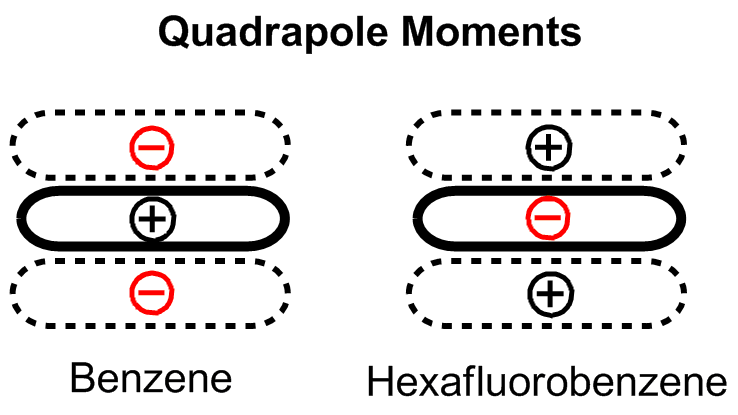
An early model for the role of substituents in pi stacking interactions was proposed by Hunter and Sanders. They used a simple mathematical model based on sigma and pi atomic charges, relative orientations, and van der Waals interactions to qualitatively determine that electrostatics are dominant
Substituent effects
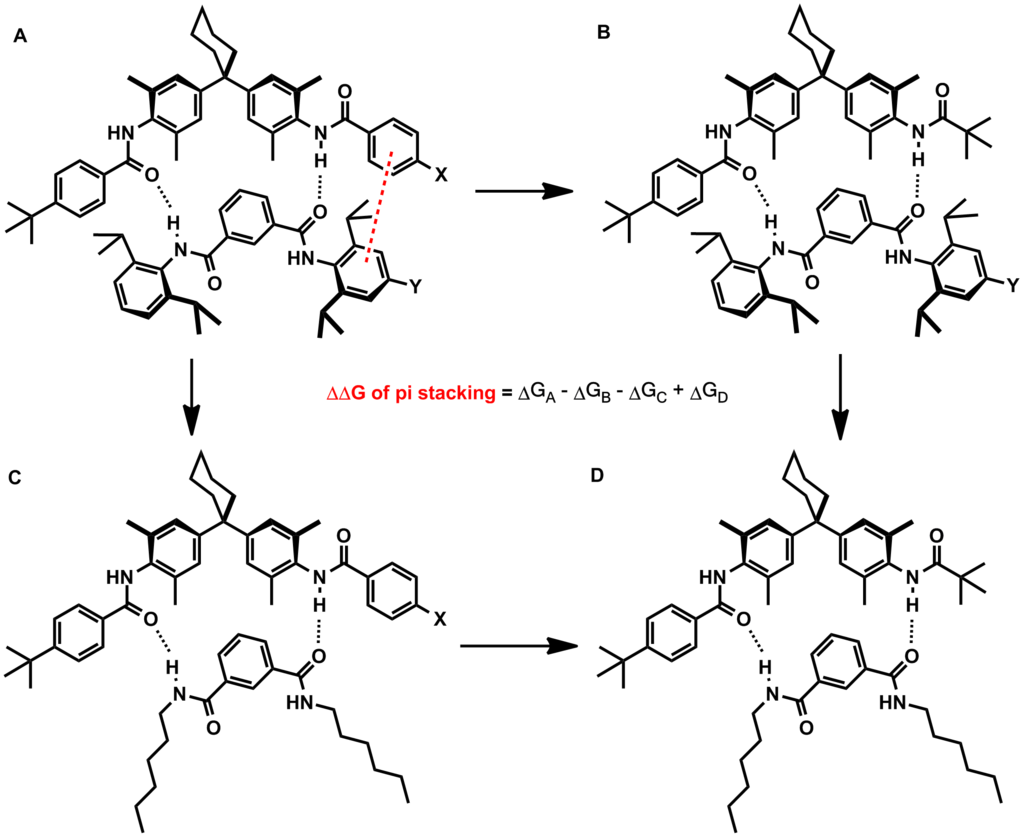
The ability to fine-tune pi stacking interactions would be useful in numerous synthetic efforts. One example would be to increase the binding affinity of a small-molecule inhibitor to an enzyme pocket containing aromatic residues. The effects of heteroatoms and substituents on pi stacking interacti
Geometric configurations
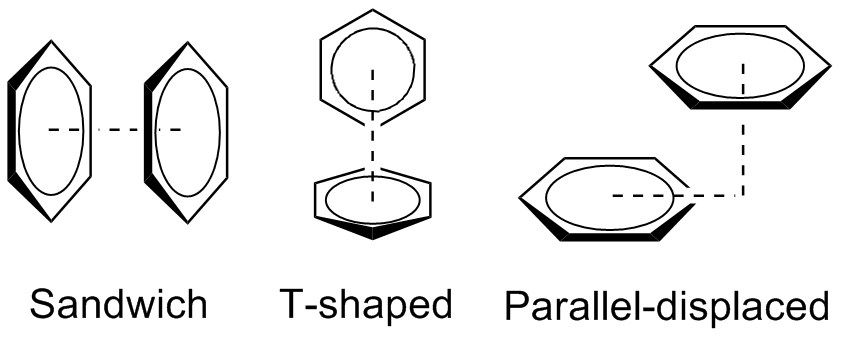
The preferred geometries of the benzene dimer have been modeled at a high level of theory with MP2-R12/A computations and very large counterpoise-corrected aug-cc-PVTZ basis sets. The two most stable conformations are the parallel displaced and T-shaped, which are essentially isoenergetic. In contra
Evidence against pi stacking
The benzene dimer is the prototypical system for the study of pi stacking, and is experimentally bound by 8–12 kJ/mol (2–3 kcal/mol) in the gas phase with a separation of 4.96 Å between the centers of mass for the T-shaped dimer. The small binding energy makes the benzene dimer difficult to s
Pi stacking aka π–π stacking
In chemistry, pi stacking (also called π–π stacking) refers to the presumptive attractive, noncovalent pi interactions (orbital overlap) between the pi bonds of aromatic rings. However this is a misleading description of the phenomena since direct stacking of aromatic rings (the R