Tag: carbon
-
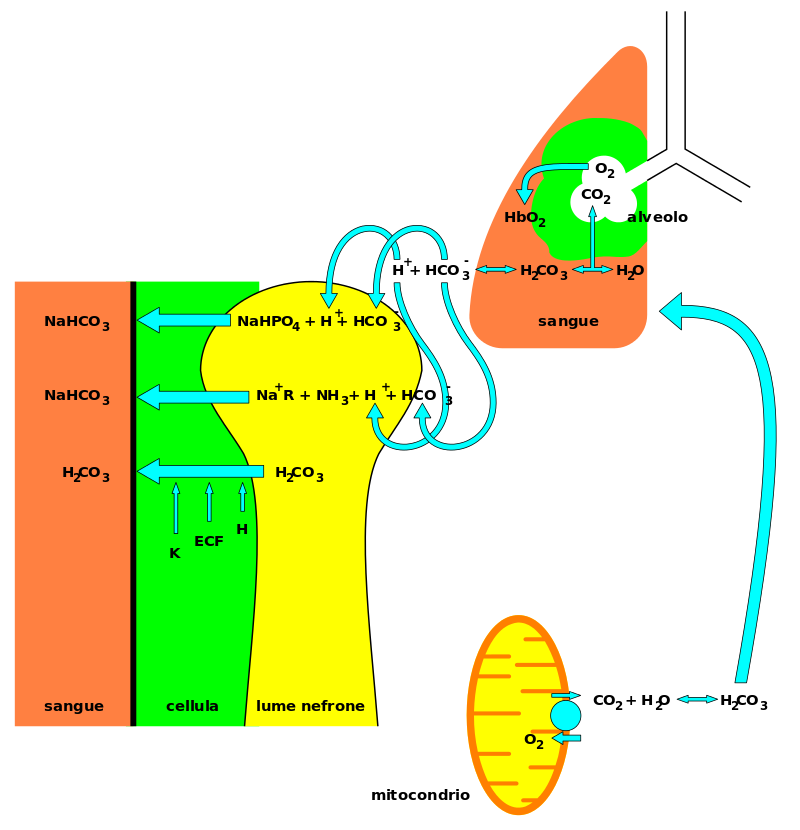
HCO3 (bicarbonate)
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula HCO3. Bicarbonate serves a crucial biochemical role in the physiological pH buffering system. The term “bicarbonate” was coined in 1814 by the English chemist William Hyde Wollaston. The name lives on as a trivial name. Chemical properties The bicarbonate ion (hydrogencarbonate ion) is an anion with the empirical formula HCO−3 and a molecular…
-
Conductive ink
Conductive ink is an ink that results in a printed object which conducts electricity. It is typically created by infusing graphite or other conductive materials into ink. There has been a growing interest in replacing metallic materials with nanomaterials due to the emergence of nanotechnology. Among other nanomaterials, graphene, and carbon nanotube-based conductive ink are gaining immense popularity due to their high…
-

Black snake (firework)
“Black snake” is a term that can refer to two similar types of fireworks: the Pharaoh’s snake and the sugar snake. The “Pharaoh’s snake” or “Pharaoh’s serpent” is the original version of the black snake experiment. It produces a more impressive snake, but its execution depends upon mercury (II) thiocyanate, which is no longer in common use due…
-

Carbon snake
Carbon snake is a demonstration of the dehydration reaction of sugar by concentrated sulfuric acid. With concentrated sulfuric acid, granulated table sugar (sucrose) performs a degradation reaction which changes its form to a black solid-liquid mixture. The carbon snake experiment can sometimes be misidentified as the black snake, “sugar snake”, or “burning sugar” reaction, all of which involve baking soda rather than sulfuric acid. Explanation Concentrated…
Recent Posts
- 🧬 Disease Table with Low Sodium Connection
- 🧂 Sodium Reduction and Sodium Replacement: A History of Reformulation and Exploding Diseases, Including Many Diseases Unheard of Before Deadly Sodium Policies
- 🧂 The DEADLY 1500 mg Sodium Recommendation predates the WHO’s formal global sodium reduction push by nearly a decade (and it’s even worse than that)
- 🧬 What Is Beta-Glucuronidase?
- When Sugar Was Salt: Crystalline Confusion and the Covenant of Sweetness
Tags
ADAM ASPARTAME Birds Blood Bones Brain Bugs Cancer Columba Cows crystallography Death Death cults Eggs Etymology Gastrin Gold Growth hormone History Hormones Insulin Liver Mere Perplexity Metal Monkey Business Mythology Paracetamol Plants Poison Pregnancy Protein Religion Reproduction Rocks Salt Slavery Snakes Sodium the birds and the bees Thiocyanate Tobacco Tylenol Underworld Venom zinc