Microfold cells (or M cells) are found in the gut-associated lymphoid tissue (GALT) of the Peyer’s patches in the small intestine, and in the mucosa-associated lymphoid tissue (MALT) of other parts of the gastrointestinal tract. These cells are known to initiate mucosal immunity responses on the apical membrane of the M cells and allow for transport of microbes and particles across the epithelial cell layer from the gut lumen to the lamina propria where interactions with immune cells can take place.
- Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A (July 2013). “Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium”. Mucosal Immunology. 6 (4): 666–677. doi:10.1038/mi.2013.30. PMC 3686595. PMID 23695511
Unlike their neighbor cells, M cells have the unique ability to take up antigen from the lumen of the small intestine via endocytosis, phagocytosis, or transcytosis. Antigens are delivered to antigen-presenting cells, such as dendritic cells, and B lymphocytes. M cells express the protease cathepsin E, similar to other antigen-presenting cells. This process takes place in a unique pocket-like structure on their basolateral side. Antigens are recognized via expression of cell surface receptors such as glycoprotein-2 (GP2) that detect and specifically bind to bacteria. Cellular prion protein (PrP) is another example of a cell surface receptor on M cells.
- Miller H, Zhang J, Kuolee R, Patel GB, Chen W (March 2007). “Intestinal M cells: the fallible sentinels?”. World Journal of Gastroenterology. 13 (10): 1477–1486. doi:10.3748/wjg.v13.i10.1477. PMC 1876659. PMID 17461437.
Major prion protein (PrP) is encoded in the human body by the PRNP gene also known as CD230 (cluster of differentiation 230). Expression of the protein is most predominant in the nervous system but occurs in many other tissues throughout the body. The protein can exist in multiple isoforms: the normal PrPC form, and the protease-resistant form designated PrPRes such as the disease-causing PrPSc (scrapie) and an isoform located in mitochondria. The misfolded version PrPSc is associated with a variety of cognitive disorders and neurodegenerative diseases such as in animals: ovine scrapie, bovine spongiform encephalopathy (BSE, mad cow disease), feline spongiform encephalopathy, transmissible mink encephalopathy (TME), exotic ungulate encephalopathy, chronic wasting disease (CWD) which affects deer; and in humans: Creutzfeldt–Jakob disease (CJD), fatal familial insomnia (FFI), Gerstmann–Sträussler–Scheinker syndrome (GSS), kuru, and variant Creutzfeldt–Jakob disease (vCJD). Similarities exist between kuru, thought to be due to human ingestion of diseased individuals, and vCJD, thought to be due to human ingestion of BSE-tainted cattle products. (Not to be confused with prions, infectious forms of proteins which have so far been observed in almost all instances to be forms of PRNP, but need not be.)
- Kretzschmar HA, Stowring LE, Westaway D, Stubblebine WH, Prusiner SB, Dearmond SJ (August 1986). “Molecular cloning of a human prion protein cDNA”. DNA. 5 (4): 315–24. doi:10.1089/dna.1986.5.315. PMID 3755672.
- Sparkes RS, Simon M, Cohn VH, Fournier RE, Lem J, Klisak I, Heinzmann C, Blatt C, Lucero M, Mohandas T (October 1986). “Assignment of the human and mouse prion protein genes to homologous chromosomes”. Proc. Natl. Acad. Sci. U.S.A. 83 (19): 7358–62. Bibcode:1986PNAS…83.7358S. doi:10.1073/pnas.83.19.7358. PMC 386716. PMID 3094007.
- Liao YC, Lebo RV, Clawson GA, Smuckler EA (July 1986). “Human prion protein cDNA: molecular cloning, chromosomal mapping, and biological implications”. Science. 233 (4761): 364–7. Bibcode:1986Sci…233..364L. doi:10.1126/science.3014653. PMID 3014653.
- Robakis NK, Devine-Gage EA, Jenkins EC, Kascsak RJ, Brown WT, Krawczun MS, Silverman WP (October 1986). “Localization of a human gene homologous to the PrP gene on the p arm of chromosome 20 and detection of PrP-related antigens in normal human brain”. Biochem. Biophys. Res. Commun. 140 (2): 758–65. doi:10.1016/0006-291X(86)90796-5. PMID 2877664.
- Prusiner SB (2001). “Shattuck lecture–neurodegenerative diseases and prions”. N Engl J Med. 344 (20): 1516–26. doi:10.1056/NEJM200105173442006. PMID 11357156.
- Weissmann C (2004). “The state of the prion”. Nat Rev Microbiol. 2 (11): 861–71. doi:10.1038/nrmicro1025. PMID 15494743. S2CID 20992257.
- Zomosa-Signoret V, Arnaud JD, Fontes P, Alvarez-Martinez MT, Liautard JP (2008). “Physiological role of the cellular prion protein” (PDF). Vet. Res. 39 (4): 9. doi:10.1051/vetres:2007048. PMID 18073096.
M cells lack microvilli but, like other epithelial cells, they are characterized by strong cell junctions. This provides a physical barrier that constitutes an important line of defense between the gut contents and the immune system of the host. Despite the epithelial barrier, some antigens are able to infiltrate the M cell barrier and infect the nearby epithelial cells or enter the gut.
- Kanaya T, Ohno H (2014). “The Mechanisms of M-cell Differentiation”. Bioscience of Microbiota, Food and Health. 33 (3): 91–97. doi:10.12938/bmfh.33.91. PMC 4098651. PMID 25032083.
Structure
M cells are distinguished from other intestinal epithelial cells by their morphological differences. They are characterized by their short microvilli or lack of these protrusions on the cell surface. When they present microvilli, they are short, irregular, and present on the apical surface or pocket-like invagination on the basolateral surface of these cells. When they lack microvilli, they are characterized by their microfolds, and hence receive their commonly known name. These cells are far less abundant than enterocytes. These cells can also be identified by cytoskeletal and extracellular matrix components expressed at the edge of cells or on their cell surfaces, such as actin, villin, cytokeratin, and vimentin.
- Kanaya T, Ohno H (2014). “The Mechanisms of M-cell Differentiation”. Bioscience of Microbiota, Food and Health. 33 (3): 91–97. doi:10.12938/bmfh.33.91. PMC 4098651. PMID 25032083.
Villin-1 is a 92.5 kDa tissue-specific actin-binding protein associated with the actin core bundle of the brush border. Villin-1 is encoded by the VIL1 gene. Villin-1 contains multiple gelsolin-like domains capped by a small (8.5 kDa) “headpiece” at the C-terminus consisting of a fast and independently folding three-helix bundle that is stabilized by hydrophobic interactions. The headpiece domain is a commonly studied protein in molecular dynamics due to its small size and fast folding kinetics and short primary sequence. See also Supervillin
- Friederich E, Vancompernolle K, Louvard D, Vandekerckhove J (September 1999). “Villin function in the organization of the actin cytoskeleton. Correlation of in vivo effects to its biochemical activities in vitro”. The Journal of Biological Chemistry. 274 (38): 26751–60. doi:10.1074/jbc.274.38.26751. PMID 10480879.
- Ghoshdastider U, Popp D, Burtnick LD, Robinson RC (November 2013). “The expanding superfamily of gelsolin homology domain proteins”. Cytoskeleton. 70 (11): 775–95. doi:10.1002/cm.21149. PMID 24155256. S2CID 205643538.
- Bazari WL, Matsudaira P, Wallek M, Smeal T, Jakes R, Ahmed Y (July 1988). “Villin sequence and peptide map identify six homologous domains”. Proceedings of the National Academy of Sciences of the United States of America. 85 (14): 4986–90. Bibcode:1988PNAS…85.4986B. doi:10.1073/pnas.85.14.4986. PMC 281672. PMID 2839826.
- Klahre U, Friederich E, Kost B, Louvard D, Chua NH (January 2000). “Villin-like actin-binding proteins are expressed ubiquitously in Arabidopsis”. Plant Physiology. 122 (1): 35–48. doi:10.1104/pp.122.1.35. PMC 58842. PMID 10631247.
Cytokeratins are keratin proteins found in the intracytoplasmic cytoskeleton of epithelial tissue. They are an important component of intermediate filaments, which help cells resist mechanical stress. Expression of these cytokeratins within epithelial cells is largely specific to particular organs or tissues. Thus they are used clinically to identify the cell of origin of various human tumors. The term cytokeratin began to be used in the late 1970s, when the protein subunits of keratin intermediate filaments inside cells were first being identified and characterized. In 2006 a new systematic nomenclature for mammalian keratins was created, and the proteins previously called cytokeratins are simply called keratins (human epithelial category). For example, cytokeratin-4 (CK-4) has been renamed keratin-4 (K4). However, they are still commonly referred to as cytokeratins in clinical practice. There are two categories of cytokeratins: the acidic type I cytokeratins and the basic or neutral type II cytokeratins.
- Herrmann H, Bär H, Kreplak L, Strelkov SV, Aebi U (July 2007). “Intermediate filaments: from cell architecture to nanomechanics”. Nat. Rev. Mol. Cell Biol. 8 (7): 562–73. doi:10.1038/nrm2197. PMID 17551517. S2CID 27115011.
- Franke WW, Schmid E, Osborn M, Weber K (June 1979). “Intermediate-sized filaments of human endothelial cells”. The Journal of Cell Biology. 81 (3): 570–80. doi:10.1083/jcb.81.3.570. PMC 2110384. PMID 379021.
- Schweizer J, Bowden PE, Coulombe PA, et al. (July 2006). “New consensus nomenclature for mammalian keratins”. The Journal of Cell Biology. 174 (2): 169–74. doi:10.1083/jcb.200603161. PMC 2064177. PMID 16831889.
- Rekhtman, Natasha; Bishop, Justin A. (2011). Quick Reference Handbook for Surgical Pathologists. Heidelberg: Springer. pp. 4–8. ISBN 978-3-642-20085-4.
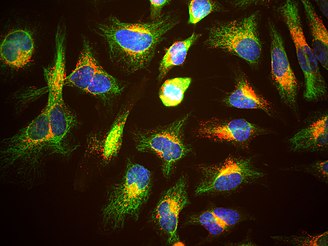
Vimentin is a structural protein that in humans is encoded by the VIM gene. Its name comes from the Latin vimentum which refers to an array of flexible rods. Vimentin is a type III intermediate filament (IF) protein that is expressed in mesenchymal cells. IF proteins are found in all animal cells as well as bacteria. Intermediate filaments, along with tubulin-based microtubules and actin-based microfilaments, comprises the cytoskeleton. All IF proteins are expressed in a highly developmentally-regulated fashion; vimentin is the major cytoskeletal component of mesenchymal cells. Because of this, vimentin is often used as a marker of mesenchymally-derived cells or cells undergoing an epithelial-to-mesenchymal transition (EMT) during both normal development and metastatic progression. It has been used as a sarcoma tumor marker to identify mesenchyme. Its specificity as a biomarker has been disputed by Jerad Gardner. Methylation of the vimentin gene has been established as a biomarker of colon cancer and this is being utilized in the development of fecal tests for colon cancer. Statistically significant levels of vimentin gene methylation have also been observed in certain upper gastrointestinal pathologies such as Barrett’s esophagus, esophageal adenocarcinoma, and intestinal type gastric cancer. High levels of DNA methylation in the promoter region have also been associated with markedly decreased survival in hormone positive breast cancers. Downregulation of vimentin was identified in cystic variant of papillary thyroid carcinoma using a proteomic approach. See also Anti-citrullinated protein antibody for its use in diagnosis of rheumatoid arthritis. Vimentin was discovered to be an attachment factor for SARS-CoV-2 by Nader Rahimi and colleagues.
- Franke WW, Schmid E, Osborn M, Weber K (October 1978). “Different intermediate-sized filaments distinguished by immunofluorescence microscopy”. Proceedings of the National Academy of Sciences of the United States of America. 75 (10): 5034–5038. Bibcode:1978PNAS…75.5034F. doi:10.1073/pnas.75.10.5034. PMC 336257. PMID 368806.
- Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD (July 2009). “Introducing intermediate filaments: from discovery to disease”. The Journal of Clinical Investigation. 119 (7): 1763–1771. doi:10.1172/JCI38339. PMC 2701876. PMID 19587451.
- Cabeen MT, Jacobs-Wagner C (2010). “The bacterial cytoskeleton”. Annual Review of Genetics. 44: 365–392. doi:10.1146/annurev-genet-102108-134845. PMID 21047262.
- Leader M, Collins M, Patel J, Henry K (January 1987). “Vimentin: an evaluation of its role as a tumour marker”. Histopathology. 11 (1): 63–72. doi:10.1111/j.1365-2559.1987.tb02609.x. PMID 2435649. S2CID 34804720.
- “Immunohistochemistry from the Washington Animal Disease Diagnostic laboratory (WADDL)of the College of Veterinary Medicine, Washington State University”. Archived from the original on 2008-12-01. Retrieved 2009-03-14.
- Gardner J (23 September 2010). “How to Interpret Vimentin Immunostain”. YouTube. Archived from the original on 2021-12-12.
- Moinova H, Leidner RS, Ravi L, Lutterbaugh J, Barnholtz-Sloan JS, Chen Y, et al. (April 2012). “Aberrant vimentin methylation is characteristic of upper gastrointestinal pathologies”. Cancer Epidemiology, Biomarkers & Prevention. 21 (4): 594–600. doi:10.1158/1055-9965.EPI-11-1060. PMC 3454489. PMID 22315367.
- Ulirsch J, Fan C, Knafl G, Wu MJ, Coleman B, Perou CM, Swift-Scanlan T (January 2013). “Vimentin DNA methylation predicts survival in breast cancer”. Breast Cancer Research and Treatment. 137 (2): 383–396. doi:10.1007/s10549-012-2353-5. PMC 3838916. PMID 23239149.
- Dinets A, Pernemalm M, Kjellin H, Sviatoha V, Sofiadis A, Juhlin CC, et al. (2015). “Differential protein expression profiles of cyst fluid from papillary thyroid carcinoma and benign thyroid lesions”. PLOS ONE. 10 (5): e0126472. Bibcode:2015PLoSO..1026472D. doi:10.1371/journal.pone.0126472. PMC 4433121. PMID 25978681.
- Amraei R, Xia C, Olejnik J, White MR, Napoleon MA, Lotfollahzadeh S, et al. (February 2022). “Extracellular vimentin is an attachment factor that facilitates SARS-CoV-2 entry into human endothelial cells”. Proceedings of the National Academy of Sciences of the United States of America. 119 (6). doi:10.1073/pnas.2113874119. PMC 8833221. PMID 35078919. e2113874119.
Development
Factors promoting the differentiation of M cells have yet to be elucidated, but they are thought to develop in response to signals from immune cells found in developing Peyer’s patches. B cells have been implicated in the developmental of M cells, since they are also localized in high numbers in the follicular-associated epithelium (FAE). FAE lacking B cell populations results in a decrease in the number of M cell lining the Peyer’s patches. Similarly, a human lymphoma cell line is also known to undergo transition from adenocarcinoma cells to M cells.
- Kraehenbuhl JP, Neutra MR (2000). “Epithelial M cells: differentiation and function”. Annual Review of Cell and Developmental Biology. 16: 301–332. doi:10.1146/annurev.cellbio.16.1.301. PMID 11031239. Link
Though many studies have shown various cell types directing the differentiation of M cells, new research characterizes the molecular pathways that guide M cell differentiation. More recently, through loss-of-function and rescue-phenotype studies, RANKL is shown to be a receptor activator of NF-κB ligand and play a role in differentiation of M cells. RANKL is expressed throughout the small intestine, facilitates uptake of pathogens such as Salmonella, and is the most critical factor M cell differentiation. Microbes found on intestinal epithelium are known to direct M cell development. For example, the type III secretion system effector protein SopB activates the transition of M cells from enterocytes. M cells undergo the differentiation process for up to four days before reaching full maturation. Recent studies have suggested they arise distinctly from the lymphoid and myeloid lineages.
- Knoop KA, Kumar N, Butler BR, Sakthivel SK, Taylor RT, Nochi T, et al. (November 2009). “RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium”. Journal of Immunology. 183 (9): 5738–5747. doi:10.4049/jimmunol.0901563. PMC 2922944. PMID 19828638.
- Tahoun A, Mahajan S, Paxton E, Malterer G, Donaldson DS, Wang D, et al. (November 2012). “Salmonella transforms follicle-associated epithelial cells into M cells to promote intestinal invasion”. Cell Host & Microbe. 12 (5): 645–656. doi:10.1016/j.chom.2012.10.009. PMID 23159054.
- Ohno H, Kanaya T, Williams IR (November 2012). “M cell differentiation: distinct lineage or phenotypic transition? Salmonella provides answers”. Cell Host & Microbe. 12 (5): 607–609. doi:10.1016/j.chom.2012.11.003. PMID 23159049.
Receptor activator of nuclear factor kappa-Β ligand (RANKL), also known as tumor necrosis factor ligand superfamily member 11 (TNFSF11), TNF-related activation-induced cytokine (TRANCE), osteoprotegerin ligand (OPGL), and osteoclast differentiation factor (ODF), is a protein that in humans is encoded by the TNFSF11 gene. RANKL is known as a type II membrane protein and is a member of the tumor necrosis factor (TNF) superfamily. RANKL has been identified to affect the immune system and control bone regeneration and remodeling. RANKL is an apoptosis regulator gene, a binding partner of osteoprotegerin (OPG), a ligand for the receptor RANK and controls cell proliferation by modifying protein levels of Id4, Id2 and cyclin D1. RANKL is expressed in several tissues and organs including: skeletal muscle, thymus, liver, colon, small intestine, adrenal gland, osteoblast, mammary gland epithelial cells, prostate and pancreas. Variation in concentration levels of RANKL throughout several organs reconfirms the importance of RANKL in tissue growth (particularly bone growth) and immune functions within the body.
- Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, Frankel WN, Lee SY, Choi Y (October 1997). “TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells”. J. Biol. Chem. 272 (40): 25190–4. doi:10.1074/jbc.272.40.25190. PMID 9312132.
- Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L (November 1997). “A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function”. Nature. 390 (6656): 175–9. Bibcode:1997Natur.390..175A. doi:10.1038/36593. PMID 9367155. S2CID 4373990.
- Hanada R, Hanada T, Sigl V, Schramek D, Penninger JM (2011). “RANKL/RANK-beyond bones”. J. Mol. Med. 89 (7): 647–56. doi:10.1007/s00109-011-0749-z. PMID 21445556. S2CID 25285776.
- Mueller CG, Hess E (2012). “Emerging Functions of RANKL in Lymphoid Tissues”. Front Immunol. 3: 261. doi:10.3389/fimmu.2012.00261. PMC 3432452. PMID 22969763.
- Wada T, Nakashima T, Hiroshi N, Penninger JM (2006). “RANKL-RANK signaling in osteoclastogenesis and bone disease”. Trends Mol Med. 12 (1): 17–25. doi:10.1016/j.molmed.2005.11.007. PMID 16356770.
Pathogens can take advantage of cell differentiation pathways in order to invade host cells. This is done by inducing differentiation of enterocytes into M cell type in gut epithelium. In one case, the SopB effector protein mentioned above is secreted to trigger fast differentiation of enterocytes localized in the FAE by initiation of epithelial to mesenchymal transition in these cells. When SopB activates differentiation of enterocytes, it acts via the activation of the Wnt/b-catenin signaling pathway and triggers the RANKL and its receptor, implicated in regulating cell apoptosis.
- Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A (July 2013). “Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium”. Mucosal Immunology. 6 (4): 666–677. doi:10.1038/mi.2013.30. PMC 3686595. PMID 23695511.
- Tahoun A, Mahajan S, Paxton E, Malterer G, Donaldson DS, Wang D, et al. (November 2012). “Salmonella transforms follicle-associated epithelial cells into M cells to promote intestinal invasion”. Cell Host & Microbe. 12 (5): 645–656. doi:10.1016/j.chom.2012.10.009. PMID 23159054.
Function
M cells do not secrete mucus or digestive enzymes, and have a thinner glycocalyx, which allows them to have easy access to the intestinal lumen for endocytosis of antigens. The main function of M cells is the selective endocytosis of antigens, and transporting them to intraepithelial macrophages and lymphocytes, which then migrate to lymph nodes where an immune response can be initiated.
- Murphy KM (2012). Janeway’s Immunobiology (8th ed.). New York: Garland Science. ISBN 978-0-8153-4243-4.
The glycocalyx (pl.: glycocalyces or glycocalyxes), also known as the pericellular matrix and sometime cell coat, is a glycoprotein and glycolipid covering that surrounds the cell membranes of bacteria, epithelial cells, and other cells. It was described in a review article in 1970. Animal epithelial cells have a fuzz-like coating on the external surface of their plasma membranes. This viscous coating is the glycocalyx that consists of several carbohydrate moieties of membrane glycolipids and glycoproteins, which serve as backbone molecules for support. Generally, the carbohydrate portion of the glycolipids found on the surface of plasma membranes helps these molecules contribute to cell–cell recognition, communication, and intercellular adhesion. The glycocalyx is a type of identifier that the body uses to distinguish between its own healthy cells and transplanted tissues, diseased cells, or invading organisms. Included in the glycocalyx are cell-adhesion molecules that enable cells to adhere to each other and guide the movement of cells during embryonic development. The glycocalyx plays a major role in regulation of endothelial vascular tissue, including the modulation of red blood cell volume in capillaries.
- Martínez-Palomo, A. (1970). “The Surface Coats of Animal Cells”. International Review of Cytology. 29: 29–75. doi:10.1016/S0074-7696(08)60032-7. ISBN 9780123643292.
- McKinley, M. & V.D. O’Loughlin. Human Anatomy. McGraw-Hill, 2012. 3rd ed. p. 30-31.
- Saladin, Kenneth. “Anatomy & Physiology: The unity of form and function.” McGraw Hill. 5th Edition. 2010. p. 94-95
- Reitsma, Sietze. “The endothelial glycocalyx: composition, functions, and visualization.” European Journal of Physiology. 2007. Vol. 454. Num. 3. p. 345-359
Passive immunity
M cells play a role in passive immunity, or the transfer of active humoral immunity during and post pregnancy. Infants rely on antibodies specific to their mother’s intestinal antigens, which move from the mother’s gut and enter the breast milk. These antibodies are able to move into the milk supply through the lymphatic system. Even though the mechanism of this transport is not fully understood, it is hypothesized that dendritic cells and macrophages play the role of transport vehicles. In females that are not lactating, when M cells recognize antigen in the gut, they stimulate production of many Immunoglobulin A (IgA) antibodies. These antibodies are released into the gut mucosa, salivary glands, and lymph nodes. However, in females that are lactating, M cells recognize antigen and IgA is directed from the gut to the mammary gland. IgA traveling from the gut to breast milk supply is controlled by hormones, chemokines, and cytokines. Thus, the mammary gland and breast milk have critical roles alongside M cells in mucosal immune system.
- Milligan L (April 2013). “From Mother’s Gut to Milk”. International Milk Genomics Consortium. Retrieved 2019-02-20.
Clinical significance
M cells are exploited by several pathogenic gram-negative bacteria including Shigella flexneri, Salmonella typhimurium, and Yersinia pseudotuberculosis, as well as infectious prions, such as in bovine spongiform encephalitis (Mad-cow disease), as a way of penetrating the intestinal epithelium. Exploitation as a virulence factor depends upon the pathogen’s ability to bind to M cells and thus guarantee penetration in that manner, as M cells sample intestinal contents. EPEC (see Pathogenic Escherichia coli) containing plasmids with genes for EAF (Escherichia coli adherence factor) will adhere to M cells. They are also exploited by viruses such as Polio and Reovirus for dissemination. CXCR4 tropic but not CCR5 tropic HIV has been noted to be able to bind to M cells and get transported across the epithelium by them.
- Ouzilou L, Caliot E, Pelletier I, Prévost MC, Pringault E, Colbère-Garapin F (September 2002). “Poliovirus transcytosis through M-like cells”. The Journal of General Virology. 83 (Pt 9): 2177–2182. doi:10.1099/0022-1317-83-9-2177. PMID 12185271.
- Fotopoulos G, Harari A, Michetti P, Trono D, Pantaleo G, Kraehenbuhl JP (July 2002). “Transepithelial transport of HIV-1 by M cells is receptor-mediated”. Proceedings of the National Academy of Sciences of the United States of America. 99 (14): 9410–9414. Bibcode:2002PNAS…99.9410F. doi:10.1073/pnas.142586899. PMC 123154. PMID 12093918.
See also
References
- Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A (July 2013). “Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium”. Mucosal Immunology. 6 (4): 666–677. doi:10.1038/mi.2013.30. PMC 3686595. PMID 23695511.
- Miller H, Zhang J, Kuolee R, Patel GB, Chen W (March 2007). “Intestinal M cells: the fallible sentinels?”. World Journal of Gastroenterology. 13 (10): 1477–1486. doi:10.3748/wjg.v13.i10.1477. PMC 1876659. PMID 17461437.
- Kanaya T, Ohno H (2014). “The Mechanisms of M-cell Differentiation”. Bioscience of Microbiota, Food and Health. 33 (3): 91–97. doi:10.12938/bmfh.33.91. PMC 4098651. PMID 25032083.
- Kraehenbuhl JP, Neutra MR (2000). “Epithelial M cells: differentiation and function”. Annual Review of Cell and Developmental Biology. 16: 301–332. doi:10.1146/annurev.cellbio.16.1.301. PMID 11031239. Link
- Knoop KA, Kumar N, Butler BR, Sakthivel SK, Taylor RT, Nochi T, et al. (November 2009). “RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium”. Journal of Immunology. 183 (9): 5738–5747. doi:10.4049/jimmunol.0901563. PMC 2922944. PMID 19828638.
- Tahoun A, Mahajan S, Paxton E, Malterer G, Donaldson DS, Wang D, et al. (November 2012). “Salmonella transforms follicle-associated epithelial cells into M cells to promote intestinal invasion”. Cell Host & Microbe. 12 (5): 645–656. doi:10.1016/j.chom.2012.10.009. PMID 23159054.
- Ohno H, Kanaya T, Williams IR (November 2012). “M cell differentiation: distinct lineage or phenotypic transition? Salmonella provides answers”. Cell Host & Microbe. 12 (5): 607–609. doi:10.1016/j.chom.2012.11.003. PMID 23159049.
- Tahoun A, Mahajan S, Paxton E, Malterer G, Donaldson DS, Wang D, et al. (November 2012). “Salmonella transforms follicle-associated epithelial cells into M cells to promote intestinal invasion”. Cell Host & Microbe. 12 (5): 645–656. doi:10.1016/j.chom.2012.10.009. PMID 23159054.
- Murphy KM (2012). Janeway’s Immunobiology (8th ed.). New York: Garland Science. ISBN 978-0-8153-4243-4.
- Milligan L (April 2013). “From Mother’s Gut to Milk”. International Milk Genomics Consortium. Retrieved 2019-02-20.
- Ouzilou L, Caliot E, Pelletier I, Prévost MC, Pringault E, Colbère-Garapin F (September 2002). “Poliovirus transcytosis through M-like cells”. The Journal of General Virology. 83 (Pt 9): 2177–2182. doi:10.1099/0022-1317-83-9-2177. PMID 12185271.
- Fotopoulos G, Harari A, Michetti P, Trono D, Pantaleo G, Kraehenbuhl JP (July 2002). “Transepithelial transport of HIV-1 by M cells is receptor-mediated”. Proceedings of the National Academy of Sciences of the United States of America. 99 (14): 9410–9414. Bibcode:2002PNAS…99.9410F. doi:10.1073/pnas.142586899. PMC 123154. PMID 12093918.
External links
| Anatomy of the gastrointestinal tract, excluding the mouth |
|---|

Leave a Reply