Category: Amino Acids
-
Protein phosphorylation was first reported in 1906
Protein phosphorylation is a reversible post-translational modification of proteins in which an amino acid residue is phosphorylated by a protein kinase by the addition of a covalently bound phosphate group. Phosphorylation alters the structural conformation of a protein, causing it to become activated, deactivated, or otherwise modifying its function. Approximately 13,000 human proteins have sites that are phosphorylated. The reverse reaction of phosphorylation is called dephosphorylation, and is catalyzed by…
-
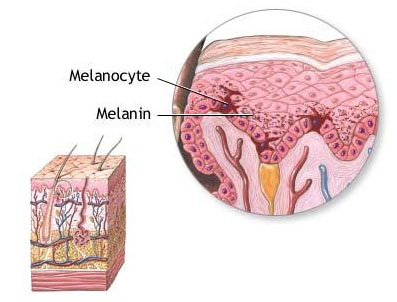
Cells in the APUD system may include melanocytes
Melanocytes are melanin-producing neural crest-derived cells located in the bottom layer (the stratum basale) of the skin’s epidermis, the middle layer of the eye (the uvea), the inner ear, vaginal epithelium, meninges, bones, and heart. Melanin is a dark pigment primarily responsible for skin color. Once synthesized, melanin is contained in special organelles called melanosomes which can be transported to nearby keratinocytes to induce pigmentation. Thus darker skin tones have more melanosomes present than lighter skin tones. Functionally, melanin serves as protection…
-
β-Methylamino-L-alanine, or BMAA
β-Methylamino-L-alanine, or BMAA, is a non-proteinogenic amino acid produced by cyanobacteria. BMAA is a neurotoxin and its potential role in various neurodegenerative disorders is the subject of scientific research. Structure and properties BMAA is a derivative of the amino acid alanine with a methylamino group on the side chain. This non-proteinogenic amino acid is classified as a polar base. Sources and detection BMAA is produced by cyanobacteria in marine, freshwater, and terrestrial environments. In…
-
Xylose is the first saccharide added to the serine or threonine in the proteoglycan type O-glycosylation
Xylose is the first saccharide added to the serine or threonine in the proteoglycan type O-glycosylation, and, so, it is the first saccharide in biosynthetic pathways of most anionic polysaccharides such as heparan sulfate and chondroitin sulfate. Definitions Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a “core protein” with one or more covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through…
-
Evidence against pi stacking
The benzene dimer is the prototypical system for the study of pi stacking, and is experimentally bound by 8–12 kJ/mol (2–3 kcal/mol) in the gas phase with a separation of 4.96 Å between the centers of mass for the T-shaped dimer. The small binding energy makes the benzene dimer difficult to study experimentally, and the dimer itself…
-
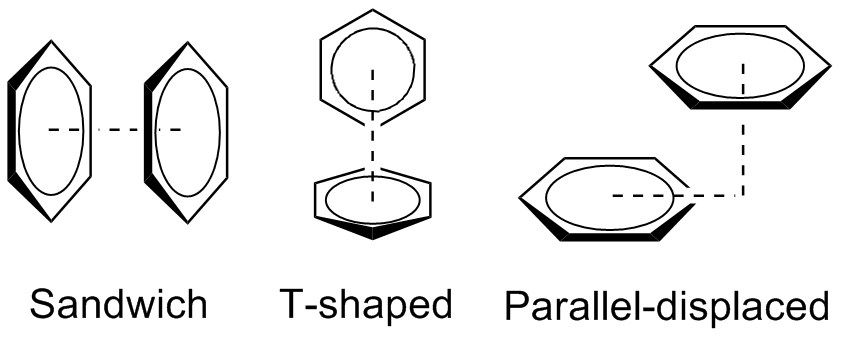
Pi stacking aka π–π stacking
In chemistry, pi stacking (also called π–π stacking) refers to the presumptive attractive, noncovalent pi interactions (orbital overlap) between the pi bonds of aromatic rings. However this is a misleading description of the phenomena since direct stacking of aromatic rings (the “sandwich interaction”) is electrostatically repulsive. What is more commonly observed (see figure below) is either a staggered stacking (parallel displaced) or pi-teeing (perpendicular T-shaped) interaction both of which are electrostatic attractive For…
-
Arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the l-arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is encoded by the codons CGU, CGC,…
NOTES
- 🧬 Disease Table with Low Sodium Connection
- 🧂 Sodium Reduction and Sodium Replacement: A History of Reformulation and Exploding Diseases, Including Many Diseases Unheard of Before Deadly Sodium Policies
- 🧂 The DEADLY 1500 mg Sodium Recommendation predates the WHO’s formal global sodium reduction push by nearly a decade (and it’s even worse than that)
- 🧬 What Is Beta-Glucuronidase?
- When Sugar Was Salt: Crystalline Confusion and the Covenant of Sweetness
Tags
ADAM ASPARTAME Birds Blood Bones Brain Bugs Cancer Columba Cows crystallography Death Death cults Eggs Etymology Gastrin Gold Growth hormone History Hormones Insulin Liver Mere Perplexity Metal Monkey Business Mythology Paracetamol Plants Poison Pregnancy Protein Religion Reproduction Rocks Salt Slavery Snakes Sodium the birds and the bees Thiocyanate Tobacco Tylenol Underworld Venom zinc